Monday Poster Session
Category: Liver
P3886 - Kratom-Induced Acute Liver Failure in a Healthy Adult
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- SP
Sheel Patel, DO
University of Louisville
Louisville, KY
Presenting Author(s)
Sheel Patel, DO, Matthew Cave, MD
University of Louisville, Louisville, KY
Introduction: Kratom is a popular, unregulated herbal supplement used for its psychotropic and opioid-like effects. Despite its widespread perception as safe, Kratom has been increasingly implicated in drug-induced liver injury (DILI), with rare reports of acute liver failure (ALF). We present a case of ALF in a previously healthy individual following Kratom use.
Case Description/
Methods: A 43-year-old female with a past medical history of plaque psoriasis presented to the emergency room with confusion, nausea, and jaundice for the past week. She reported drinking multiple bottles of Kratom daily for chronic back pain for the past five months. She denied any prior liver disease, alcohol, or illicit substance use. She started drinking Kratom five months prior for her chronic back pain. Liver function tests (LFTs) were notable for a bilirubin of 23 mg/dL, ALP 1,436 U/L, AST 644 U/L, ALT 115 U/L, and INR 1.18. Physical exam was notable for jaundice with no asterixis. Ultrasound showed a non-specific finding of coarsened hepatic echotexture. MRI MRCP showed no evidence of biliary tree pathology. Liver biopsy showed severe portal and lobular hepatitis with small bile duct injury and a predominantly lymphocytic infiltrate containing plasma cells, neutrophils, and eosinophils (Fig. 1A). Liver serological workup was unremarkable for alternative etiologies of elevated liver enzymes. She was started on empiric N-acetylcysteine for three days and Budesonide 9 mg daily for one week, with improvement in LFTs. Strict stoppage of Kratom was advised. At the two-month follow-up, LFTs showed continued improvement, with bilirubin levels down to 3.9 mg/dL (Fig. 1B).
Discussion: Acute liver failure from Kratom is a rare but serious side effect. As there are over 40 metabolites in Kratom, the exact mechanism of liver injury is unclear. Unlike most reported cases with a 1–8 week latency, our patient developed peak bilirubin levels after five months, suggesting that Kratom-induced injury may follow a prolonged or subclinical course. Biopsy findings were consistent with resolving drug-induced liver injury. Notably, the patient recovered without transplantation, highlighting the potential for reversibility with early recognition. Given Kratom’s wide availability and perceived safety, clinicians should maintain a high index of suspicion, especially in younger patients presenting with unexplained liver injury and no traditional risk factors.
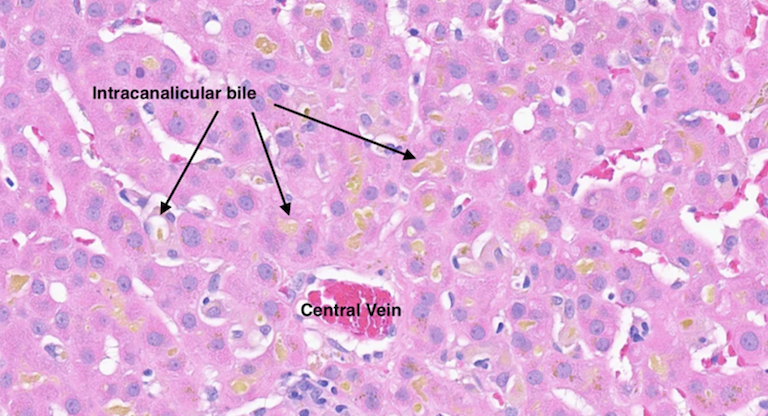
Figure: Figure 1A: Liver biopsy showing severe portal and lobular hepatitis with small bile duct injury and a predominantly lymphocytic infiltrate containing plasma cells, neutrophils, and eosinophils
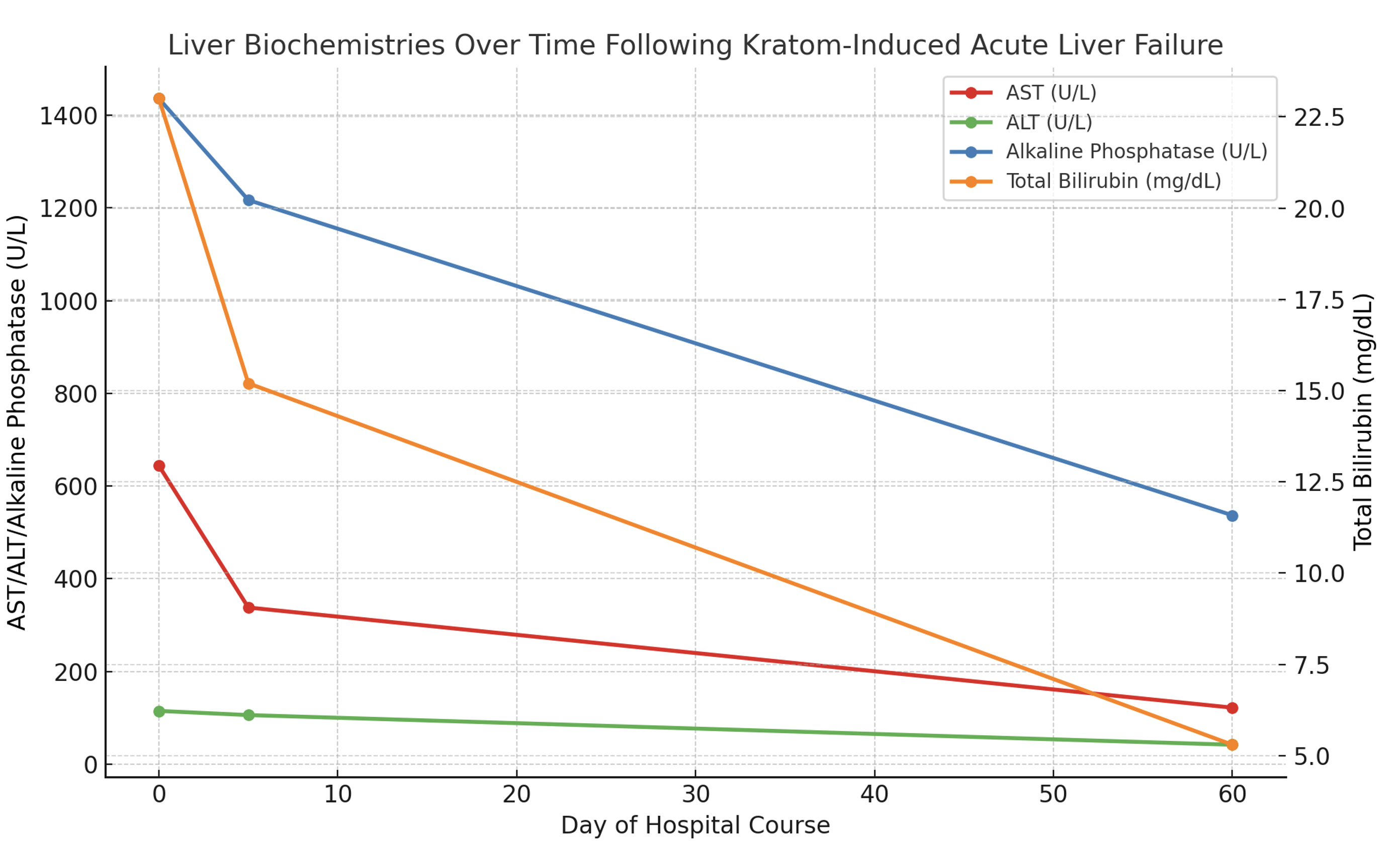
Figure: Figure 1B: Liver enzyme trend over time after treatment with N-acetylcysteine and Budesonide on day zero
Disclosures:
Sheel Patel indicated no relevant financial relationships.
Matthew Cave indicated no relevant financial relationships.
Sheel Patel, DO, Matthew Cave, MD. P3886 - Kratom-Induced Acute Liver Failure in a Healthy Adult, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Louisville, Louisville, KY
Introduction: Kratom is a popular, unregulated herbal supplement used for its psychotropic and opioid-like effects. Despite its widespread perception as safe, Kratom has been increasingly implicated in drug-induced liver injury (DILI), with rare reports of acute liver failure (ALF). We present a case of ALF in a previously healthy individual following Kratom use.
Case Description/
Methods: A 43-year-old female with a past medical history of plaque psoriasis presented to the emergency room with confusion, nausea, and jaundice for the past week. She reported drinking multiple bottles of Kratom daily for chronic back pain for the past five months. She denied any prior liver disease, alcohol, or illicit substance use. She started drinking Kratom five months prior for her chronic back pain. Liver function tests (LFTs) were notable for a bilirubin of 23 mg/dL, ALP 1,436 U/L, AST 644 U/L, ALT 115 U/L, and INR 1.18. Physical exam was notable for jaundice with no asterixis. Ultrasound showed a non-specific finding of coarsened hepatic echotexture. MRI MRCP showed no evidence of biliary tree pathology. Liver biopsy showed severe portal and lobular hepatitis with small bile duct injury and a predominantly lymphocytic infiltrate containing plasma cells, neutrophils, and eosinophils (Fig. 1A). Liver serological workup was unremarkable for alternative etiologies of elevated liver enzymes. She was started on empiric N-acetylcysteine for three days and Budesonide 9 mg daily for one week, with improvement in LFTs. Strict stoppage of Kratom was advised. At the two-month follow-up, LFTs showed continued improvement, with bilirubin levels down to 3.9 mg/dL (Fig. 1B).
Discussion: Acute liver failure from Kratom is a rare but serious side effect. As there are over 40 metabolites in Kratom, the exact mechanism of liver injury is unclear. Unlike most reported cases with a 1–8 week latency, our patient developed peak bilirubin levels after five months, suggesting that Kratom-induced injury may follow a prolonged or subclinical course. Biopsy findings were consistent with resolving drug-induced liver injury. Notably, the patient recovered without transplantation, highlighting the potential for reversibility with early recognition. Given Kratom’s wide availability and perceived safety, clinicians should maintain a high index of suspicion, especially in younger patients presenting with unexplained liver injury and no traditional risk factors.

Figure: Figure 1A: Liver biopsy showing severe portal and lobular hepatitis with small bile duct injury and a predominantly lymphocytic infiltrate containing plasma cells, neutrophils, and eosinophils

Figure: Figure 1B: Liver enzyme trend over time after treatment with N-acetylcysteine and Budesonide on day zero
Disclosures:
Sheel Patel indicated no relevant financial relationships.
Matthew Cave indicated no relevant financial relationships.
Sheel Patel, DO, Matthew Cave, MD. P3886 - Kratom-Induced Acute Liver Failure in a Healthy Adult, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
