Monday Poster Session
Category: Liver
P3850 - Drug-Induced Liver Injury With Combination Semaglutide and Atorvastatin
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ashley Robinson, DO (she/her/hers)
Advocate Lutheran General
Chicago, IL
Presenting Author(s)
Ashley Robinson, DO1, Meghana Doniparthi, MD2
1Advocate Lutheran General, Chicago, IL; 2Advocate Lutheran General, Park Ridge, IL
Introduction: Semaglutide is a glucagon-like peptide 1 (GLP-1) agonist used for management of type 2 diabetes and obesity. Adverse effects include nausea, change in bowel pattern, and rare cases of pancreatitis. Drug induced liver injury (DILI) associated with semaglutide has not been frequently described. Atorvastatin is an HMG-CoA reductase inhibitor associated with mild aminotransferase elevations in a small number of patients. We report a case of liver injury related to semaglutide and concurrent statin use.
Case Description/
Methods: A 56-year-old woman with hypertension, hyperlipidemia, type 2 diabetes and hypothyroidism was referred to the Gastroenterology clinic for evaluation of elevated liver enzymes. Initial routine labs had a normal liver panel. Oral semaglutide 3mg was started for her diabetes and increased to 7mg a month later and changed from pravastatin 40mg to atorvastatin 20mg for persistent LDL elevation. Labs after four months showed elevation of alkaline phosphatase (ALP) to 352 U/L, aspartate aminotransferase (AST) to 43 U/L and alanine aminotransferase (ALT) to 61 U/L. After two weeks ALP was 398 U/L, then 442 U/L after another two weeks. Hepatic ultrasound revealed increased hepatic echogenicity typical of steatosis. Her PCP advised her to discontinue semaglutide but continued atorvastatin. She denied personal or family history of liver disease. Her home medications include levothyroxine, losartan, metformin, and a multivitamin. She denied use of other supplements or alcohol.
Workup by GI included a negative hepatitis panel, antinuclear antibody screen, antimitochondrial antibody, iron panel and ferritin, ceruloplasmin, alpha-1 antitrypsin, and celiac testing. Anti-smooth muscle antibody mildly elevated to 1:40 and gamma-glutamyl transferase (GGT) was elevated to 349 U/L.
She remained off semaglutide with repeat liver panel two months later showing AST 20 U/L, ALT 20 U/L, and ALP normalized to 129 U/L.
Discussion: DILI associated with semaglutide is rare and though it has been well described, is uncommon with statins. Our patient had evidence of liver injury after starting semaglutide with a change of statin which improved after semaglutide was stopped despite continuation of atorvastatin. It is possible that induction of both medications concurrently caused DILI. Although rare, with the increasing popularity of GLP-1 agonists and widespread use of statins, clinicians should be aware of this potential effect and monitor hepatic function when prescribing these medications in combination.
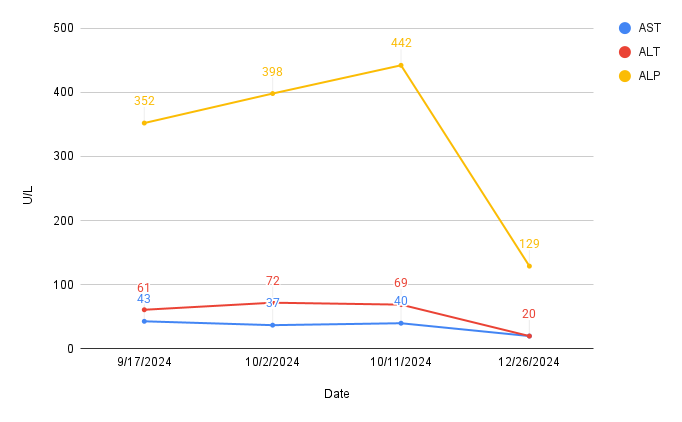
Figure: Figure 1. Trends of liver enzyme elevation following initiation of semaglutide and atorvastatin with subsequent discontinuation of semaglutide.
Disclosures:
Ashley Robinson indicated no relevant financial relationships.
Meghana Doniparthi indicated no relevant financial relationships.
Ashley Robinson, DO1, Meghana Doniparthi, MD2. P3850 - Drug-Induced Liver Injury With Combination Semaglutide and Atorvastatin, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Advocate Lutheran General, Chicago, IL; 2Advocate Lutheran General, Park Ridge, IL
Introduction: Semaglutide is a glucagon-like peptide 1 (GLP-1) agonist used for management of type 2 diabetes and obesity. Adverse effects include nausea, change in bowel pattern, and rare cases of pancreatitis. Drug induced liver injury (DILI) associated with semaglutide has not been frequently described. Atorvastatin is an HMG-CoA reductase inhibitor associated with mild aminotransferase elevations in a small number of patients. We report a case of liver injury related to semaglutide and concurrent statin use.
Case Description/
Methods: A 56-year-old woman with hypertension, hyperlipidemia, type 2 diabetes and hypothyroidism was referred to the Gastroenterology clinic for evaluation of elevated liver enzymes. Initial routine labs had a normal liver panel. Oral semaglutide 3mg was started for her diabetes and increased to 7mg a month later and changed from pravastatin 40mg to atorvastatin 20mg for persistent LDL elevation. Labs after four months showed elevation of alkaline phosphatase (ALP) to 352 U/L, aspartate aminotransferase (AST) to 43 U/L and alanine aminotransferase (ALT) to 61 U/L. After two weeks ALP was 398 U/L, then 442 U/L after another two weeks. Hepatic ultrasound revealed increased hepatic echogenicity typical of steatosis. Her PCP advised her to discontinue semaglutide but continued atorvastatin. She denied personal or family history of liver disease. Her home medications include levothyroxine, losartan, metformin, and a multivitamin. She denied use of other supplements or alcohol.
Workup by GI included a negative hepatitis panel, antinuclear antibody screen, antimitochondrial antibody, iron panel and ferritin, ceruloplasmin, alpha-1 antitrypsin, and celiac testing. Anti-smooth muscle antibody mildly elevated to 1:40 and gamma-glutamyl transferase (GGT) was elevated to 349 U/L.
She remained off semaglutide with repeat liver panel two months later showing AST 20 U/L, ALT 20 U/L, and ALP normalized to 129 U/L.
Discussion: DILI associated with semaglutide is rare and though it has been well described, is uncommon with statins. Our patient had evidence of liver injury after starting semaglutide with a change of statin which improved after semaglutide was stopped despite continuation of atorvastatin. It is possible that induction of both medications concurrently caused DILI. Although rare, with the increasing popularity of GLP-1 agonists and widespread use of statins, clinicians should be aware of this potential effect and monitor hepatic function when prescribing these medications in combination.

Figure: Figure 1. Trends of liver enzyme elevation following initiation of semaglutide and atorvastatin with subsequent discontinuation of semaglutide.
Disclosures:
Ashley Robinson indicated no relevant financial relationships.
Meghana Doniparthi indicated no relevant financial relationships.
Ashley Robinson, DO1, Meghana Doniparthi, MD2. P3850 - Drug-Induced Liver Injury With Combination Semaglutide and Atorvastatin, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
