Monday Poster Session
Category: Liver
P3800 - Low Rate of Infection-Related Adverse Events in Patients With Cirrhosis Treated With Rifaximin Soluble Solid Dispersion Tablets: An Analysis of Two Phase 2, Randomized, Double-Blind, Placebo-Controlled Trials
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- JB
Jasmohan S. Bajaj, MD, MS, FACG
Virginia Commonwealth University; Central Virginia Veterans Healthcare System
Richmond, VA
Presenting Author(s)
Jasmohan S.. Bajaj, MD, MS, FACG1, Arun J.. Sanyal, MD1, Christopher Allen, MS2, Adam P.. Laitman, MD2, Prasun K.. Jalal, MD3
1Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA; 2Salix Pharmaceuticals, Bridgewater, NJ; 3Baylor College of Medicine, Houston, TX
Introduction: Rifaximin 550-mg tablets are indicated for reducing overt hepatic encephalopathy (OHE) recurrence risk in adults. However, given the need for bile acids for maximal solubilization/activity with current rifaximin tablets, investigational rifaximin soluble solid dispersion (SSD) tablets immediate-release (IR) and sustained extended-release (SER) were developed to improve rifaximin GI luminal water solubility, while minimizing systemic exposure. Given the ongoing debate on risk of clinical antibiotic resistance in patients with cirrhosis receiving daily rifaximin, the aim was to evaluate the infection-related safety profile of rifaximin in phase 2 trials—for prevention of cirrhosis complications and as treatment of an OHE episode.
Methods: Study 1 included adults with well-controlled ascites and MELD-Na ≥12 randomized to 1 of 5 SSD groups (IR 40 mg, IR 80 mg, SER 40 mg, SER 80 mg, IR 80 mg + SER 80 mg) or placebo (PBO) once nightly for 24 wks. Study 2 had hospitalized adults with OHE randomized to PBO or 1 of 4 SSD groups (IR 40 mg once or twice daily [bid] or SER 80 mg once daily or bid) for ≤14 days.
Results: 516 (Study 1) and 71 (Study 2) patients were included. In Study 1, the overall median (range) age was 57.0 (26-83) y, 61.0% were male, and the majority (63.4%) had a MELD-Na score of 11-18; person-y of exposure ranged from 33.0 (IR 80 mg + SER 80 mg) to 38.8 (PBO). In Study 2, overall mean age was 61.4 y, 53.5% were male, 30% had a baseline HESI grade >2, and the median MELD score was 19.0; median treatment duration was 3.0 to 4.0 days across groups. Concomitant antibiotic use is in Table and infection-related adverse events (AE) in Figure. No antibiotic-resistant infections were reported as an AE in either trial. Most common infection-related AEs across SSD groups vs PBO were UTI (3.8% vs 10.6%) and pneumonia (2.1% vs 2.1%) in Study 1; UTI (3.5% vs 0%) and septic shock (3.5% vs 0%) in Study 2. In Study 1, there was also 1 AE each of Enterobacter infection (PBO), Klebsiella sepsis (IR 80 mg qhs), Salmonella bacteremia (SER 40 mg qhs), and 2 AEs each of C difficile colitis (SER 40 mg qhs) and staphylococcal infection (PBO). In Study 2, there was 1 AE of C difficile colitis (SER 80 mg bid).
Discussion: Rifaximin SSD tablets for up to 24 wks were well tolerated and did not increase the risk of developing an infection, including antibiotic-resistant infections, in patients with cirrhosis. Rifaximin SSD IR 40 mg is being evaluated in phase 3 trials that will provide further insights.
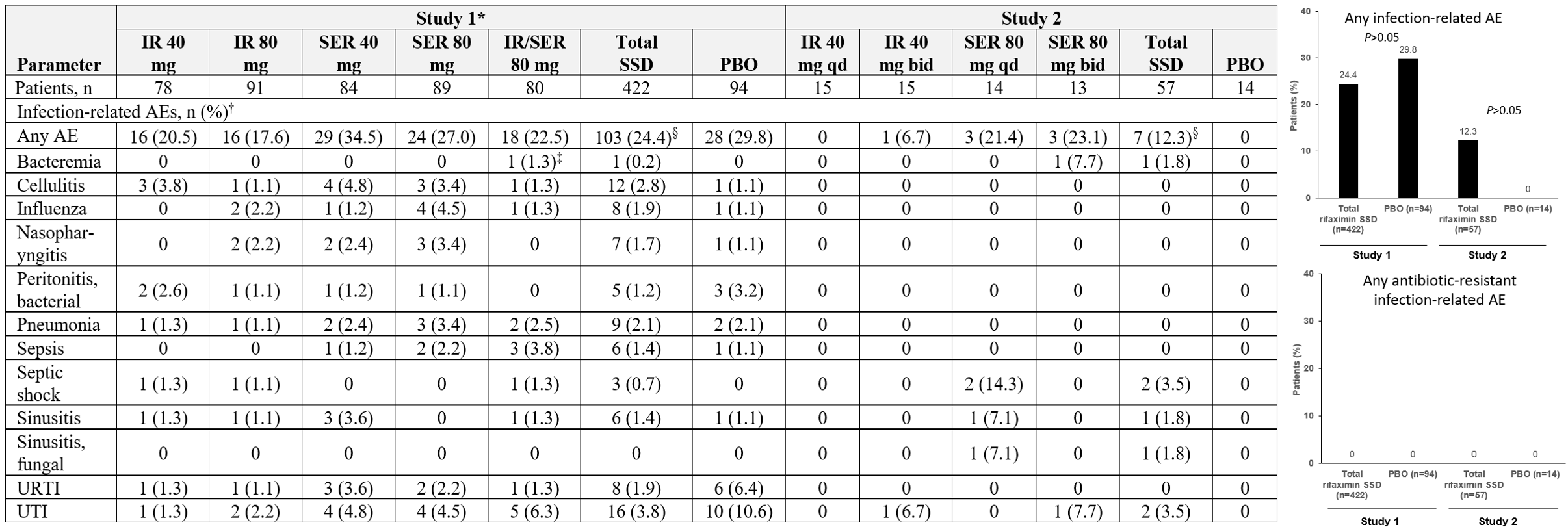
Figure: Table. Concomitant Antibiotic Use
*Administered once nightly. †Topical antibiotic use: mupirocin (n=1), bacitracin + neomycin + polymyxin B (n=2), and bacitracin zinc (n=1).
IR = immediate-release; PBO = placebo; SER = sustained extended-release.
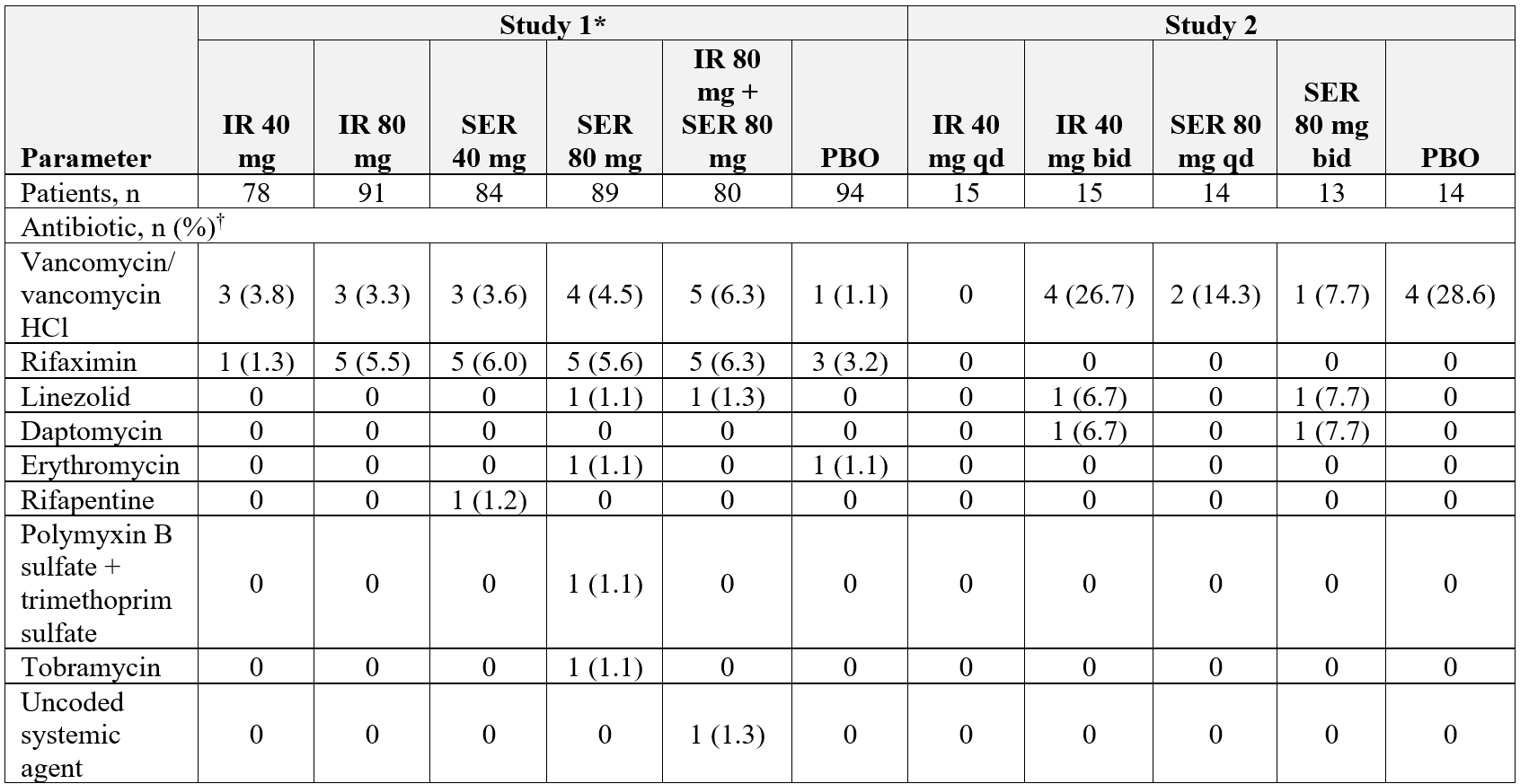
Figure: Figure. Infection-Related AEs
*Administered once nightly. †Reported in ≥1.4% of patients in Studies 1 or 2. ‡Bacteroides bacteremia. §P>0.05 total rifaximin SSD group versus PBO (Study 1, Chi-square test; Study 2, Fisher’s exact test).
AE = adverse event; bid = twice daily; IR = immediate-release; PBO = placebo; qd = once daily; SER = sustained extended-release; URTI = upper respiratory tract infection; UTI = urinary tract infection.
Disclosures:
Jasmohan Bajaj: Bausch – Grant/Research Support. Genfit – Grant/Research Support. Salix Pharmaceuticals – Clinical trial investigator. Sequana – Grant/Research Support.
Arun Sanyal: 89Bio – Advisory Committee/Board Member, Consultant. Albireo – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. AMRA – Advisory Committee/Board Member, Consultant. ARTham Therapeutics – Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Bird Rock Bio – Consultant. Blade Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Grant/Research Support. Conatus – Advisory Committee/Board Member, Consultant, Grant/Research Support. Covance – Advisory Committee/Board Member, Consultant, Grant/Research Support. Cumberland Pharmaceuticals – Grant/Research Support. CymaBay Therapeutics – Research collaborations. Durect – Stock Options. Echosens – Consultant, Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. Exhalenz – Stock Options. Fractyl – Grant/Research Support. Galmed Pharmaceuticals Ltd – Stock Options. Genentech – Advisory Committee/Board Member, Consultant. GENFIT – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant, Grant/Research Support. Glympse Bio – Consultant. Hemoshear – Advisory Committee/Board Member, Consultant, Stock Options. HistoIndex – Advisory Committee/Board Member, Consultant. Immuron – Grant/Research Support. Indalo – Stock Options. Intercept Pharmaceuticals – Grant/Research Support. Inventiva – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Labcorp – Research collaborations. Lilly – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Consultant, Grant/Research Support. Malinckrodt – Advisory Committee/Board Member, Consultant, Grant/Research Support. MedImmune – Advisory Committee/Board Member, Consultant. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Grant/Research Support. NASH Pharmaceuticals – Consultant. NGM Bio – Advisory Committee/Board Member, Consultant. NorthSea – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant, Grant/Research Support. Novo Nordisk – Advisory Committee/Board Member, Consultant, Grant/Research Support. PathAI – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Poxel – Advisory Committee/Board Member, Consultant. ProSciento – Advisory Committee/Board Member, Consultant. Regeneron Pharmaceuticals, Inc – Advisory Committee/Board Member, Consultant. Rivus – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Sanyal Bio – Stock Options. Second Genome – Research collaborations. Sequana Therapeutics – Grant/Research Support. Siemens – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Terns – Advisory Committee/Board Member, Consultant. Teva Pharmaceutical Industries Ltd – Consultant. Tiziana – Stock Options.
Christopher Allen: Salix Pharmaceuticals – Employee.
Adam Laitman: Salix Pharmaceuticals – Employee.
Prasun Jalal: AbbVie – Consultant. Gilead Sciences – Consultant.
Jasmohan S.. Bajaj, MD, MS, FACG1, Arun J.. Sanyal, MD1, Christopher Allen, MS2, Adam P.. Laitman, MD2, Prasun K.. Jalal, MD3. P3800 - Low Rate of Infection-Related Adverse Events in Patients With Cirrhosis Treated With Rifaximin Soluble Solid Dispersion Tablets: An Analysis of Two Phase 2, Randomized, Double-Blind, Placebo-Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA; 2Salix Pharmaceuticals, Bridgewater, NJ; 3Baylor College of Medicine, Houston, TX
Introduction: Rifaximin 550-mg tablets are indicated for reducing overt hepatic encephalopathy (OHE) recurrence risk in adults. However, given the need for bile acids for maximal solubilization/activity with current rifaximin tablets, investigational rifaximin soluble solid dispersion (SSD) tablets immediate-release (IR) and sustained extended-release (SER) were developed to improve rifaximin GI luminal water solubility, while minimizing systemic exposure. Given the ongoing debate on risk of clinical antibiotic resistance in patients with cirrhosis receiving daily rifaximin, the aim was to evaluate the infection-related safety profile of rifaximin in phase 2 trials—for prevention of cirrhosis complications and as treatment of an OHE episode.
Methods: Study 1 included adults with well-controlled ascites and MELD-Na ≥12 randomized to 1 of 5 SSD groups (IR 40 mg, IR 80 mg, SER 40 mg, SER 80 mg, IR 80 mg + SER 80 mg) or placebo (PBO) once nightly for 24 wks. Study 2 had hospitalized adults with OHE randomized to PBO or 1 of 4 SSD groups (IR 40 mg once or twice daily [bid] or SER 80 mg once daily or bid) for ≤14 days.
Results: 516 (Study 1) and 71 (Study 2) patients were included. In Study 1, the overall median (range) age was 57.0 (26-83) y, 61.0% were male, and the majority (63.4%) had a MELD-Na score of 11-18; person-y of exposure ranged from 33.0 (IR 80 mg + SER 80 mg) to 38.8 (PBO). In Study 2, overall mean age was 61.4 y, 53.5% were male, 30% had a baseline HESI grade >2, and the median MELD score was 19.0; median treatment duration was 3.0 to 4.0 days across groups. Concomitant antibiotic use is in Table and infection-related adverse events (AE) in Figure. No antibiotic-resistant infections were reported as an AE in either trial. Most common infection-related AEs across SSD groups vs PBO were UTI (3.8% vs 10.6%) and pneumonia (2.1% vs 2.1%) in Study 1; UTI (3.5% vs 0%) and septic shock (3.5% vs 0%) in Study 2. In Study 1, there was also 1 AE each of Enterobacter infection (PBO), Klebsiella sepsis (IR 80 mg qhs), Salmonella bacteremia (SER 40 mg qhs), and 2 AEs each of C difficile colitis (SER 40 mg qhs) and staphylococcal infection (PBO). In Study 2, there was 1 AE of C difficile colitis (SER 80 mg bid).
Discussion: Rifaximin SSD tablets for up to 24 wks were well tolerated and did not increase the risk of developing an infection, including antibiotic-resistant infections, in patients with cirrhosis. Rifaximin SSD IR 40 mg is being evaluated in phase 3 trials that will provide further insights.

Figure: Table. Concomitant Antibiotic Use
*Administered once nightly. †Topical antibiotic use: mupirocin (n=1), bacitracin + neomycin + polymyxin B (n=2), and bacitracin zinc (n=1).
IR = immediate-release; PBO = placebo; SER = sustained extended-release.

Figure: Figure. Infection-Related AEs
*Administered once nightly. †Reported in ≥1.4% of patients in Studies 1 or 2. ‡Bacteroides bacteremia. §P>0.05 total rifaximin SSD group versus PBO (Study 1, Chi-square test; Study 2, Fisher’s exact test).
AE = adverse event; bid = twice daily; IR = immediate-release; PBO = placebo; qd = once daily; SER = sustained extended-release; URTI = upper respiratory tract infection; UTI = urinary tract infection.
Disclosures:
Jasmohan Bajaj: Bausch – Grant/Research Support. Genfit – Grant/Research Support. Salix Pharmaceuticals – Clinical trial investigator. Sequana – Grant/Research Support.
Arun Sanyal: 89Bio – Advisory Committee/Board Member, Consultant. Albireo – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. AMRA – Advisory Committee/Board Member, Consultant. ARTham Therapeutics – Consultant. AstraZeneca – Advisory Committee/Board Member, Consultant. Bird Rock Bio – Consultant. Blade Therapeutics – Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant, Grant/Research Support. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Grant/Research Support. Conatus – Advisory Committee/Board Member, Consultant, Grant/Research Support. Covance – Advisory Committee/Board Member, Consultant, Grant/Research Support. Cumberland Pharmaceuticals – Grant/Research Support. CymaBay Therapeutics – Research collaborations. Durect – Stock Options. Echosens – Consultant, Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. Exhalenz – Stock Options. Fractyl – Grant/Research Support. Galmed Pharmaceuticals Ltd – Stock Options. Genentech – Advisory Committee/Board Member, Consultant. GENFIT – Advisory Committee/Board Member, Consultant, Stock Options. Gilead – Advisory Committee/Board Member, Consultant, Grant/Research Support. Glympse Bio – Consultant. Hemoshear – Advisory Committee/Board Member, Consultant, Stock Options. HistoIndex – Advisory Committee/Board Member, Consultant. Immuron – Grant/Research Support. Indalo – Stock Options. Intercept Pharmaceuticals – Grant/Research Support. Inventiva – Advisory Committee/Board Member, Consultant, Grant/Research Support. Janssen – Advisory Committee/Board Member, Consultant. Labcorp – Research collaborations. Lilly – Grant/Research Support. Madrigal – Advisory Committee/Board Member, Consultant, Grant/Research Support. Malinckrodt – Advisory Committee/Board Member, Consultant, Grant/Research Support. MedImmune – Advisory Committee/Board Member, Consultant. Merck Sharp & Dohme – Advisory Committee/Board Member, Consultant, Grant/Research Support. NASH Pharmaceuticals – Consultant. NGM Bio – Advisory Committee/Board Member, Consultant. NorthSea – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant, Grant/Research Support. Novo Nordisk – Advisory Committee/Board Member, Consultant, Grant/Research Support. PathAI – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Poxel – Advisory Committee/Board Member, Consultant. ProSciento – Advisory Committee/Board Member, Consultant. Regeneron Pharmaceuticals, Inc – Advisory Committee/Board Member, Consultant. Rivus – Stock Options. Roche – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Sanofi – Advisory Committee/Board Member, Consultant. Sanyal Bio – Stock Options. Second Genome – Research collaborations. Sequana Therapeutics – Grant/Research Support. Siemens – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. Terns – Advisory Committee/Board Member, Consultant. Teva Pharmaceutical Industries Ltd – Consultant. Tiziana – Stock Options.
Christopher Allen: Salix Pharmaceuticals – Employee.
Adam Laitman: Salix Pharmaceuticals – Employee.
Prasun Jalal: AbbVie – Consultant. Gilead Sciences – Consultant.
Jasmohan S.. Bajaj, MD, MS, FACG1, Arun J.. Sanyal, MD1, Christopher Allen, MS2, Adam P.. Laitman, MD2, Prasun K.. Jalal, MD3. P3800 - Low Rate of Infection-Related Adverse Events in Patients With Cirrhosis Treated With Rifaximin Soluble Solid Dispersion Tablets: An Analysis of Two Phase 2, Randomized, Double-Blind, Placebo-Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
