Monday Poster Session
Category: Liver
P3796 - Growing Acceptance of Live Microbial Therapeutics in Patients With Alcohol-Related Liver Disease to Reduce At-Risk Drinking
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Anas Aljabi, MD
Virginia Commonwealth University Health System
Richmond, VA
Presenting Author(s)
Anas Aljabi, MD1, Jasmohan S.. Bajaj, MD, MS, FACG2, Vinay Jahagirdar, MBBS3, Amy Bartels, RN1, Andrew Fagan, BS4, Albert Arias, MD, MS1, Alexander Khoruts, MD5
1Virginia Commonwealth University Health System, Richmond, VA; 2Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA; 3Division of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University Health System, Richmond, VA; 4Hunter Holmes McGuire VA Medical Center, Richmond, VA; 5University of Minnesota Medical Center, Minneapolis, MN
Introduction: Alcohol use disorder (AUD) and resultant liver disease (ALD) have a strong gut-liver-brain axis foundation. Recent animal and small human studies have shown that fecal microbiota transplant (FMT) can reduce problem drinking, but the acceptance of this modality in a larger group is unclear.
Aim: Determine acceptance of FMT in patients with concomitant AUD+ALD.
Methods: The Intestinal Microbiota Transplant in Alcohol-Associated Liver Disease (IMPACT) trial is studying whether capsule FMT versus placebo can enhance alcohol abstinence over 7 months in AUD+ALD patients. Recruitment is from a University and a VA hospital. Eligible subjects are age >21 yr, current alcohol use and AUD (AUDIT-10>8) with prior or current AUD therapies, ALD (FIB-4 >2.67 through cirrhosis) and able to provide stool samples. While main exclusions were food allergies, psychosis, allergies, concomitant drug use, dysphagia, hepatic encephalopathy (HE), or inability to consent. Reasons for acceptance or refusal to participate in the trial, specifically refusal due to FMT as a modality, were recorded. Comparisons between those who accepted versus declined were compared.
Results:
We evaluated 176 medical charts, of which 89 a priori did not meet the criteria (37 without ALD, 26 abstinent, 11 HE, 10 drug use, 6 other end-organ failures). The remaining 87 patients who qualified for the study were contacted, of whom 54 (62%) agreed to participate. Of the 33 who declined, 26 refused due to logistics and multiple study visits while only 7 patients refused due to discomfort with ingesting donor microbes.
Comparisons between those agreed or declined: As shown in Table 1, those who declined were more likely to be Veterans and have a higher AUDIT-10 and less likely to be on behavioral therapy than those agreed to participate. Demographics, proportion with cirrhosis, type of alcohol misuse pattern, and medication therapy for alcohol were statistically similar.
Declined due to FMT: was only seen in 8% (7 of 87) patients. All were men with cirrhosis of which 6 were Veterans. Three were on medical AUD therapy.
Discussion: In recruitment for a trial for FMT capsules in AUD+ALD who did not respond to behavioral or medical AUD therapy, most subjects approached were agreeable to participate. Most reasons for declining were related to logistics and only 8% of patients approached took issue with donor microbe ingestion. Live microbial biotherapeutic are acceptable to most AUD and ALD patients and if effective could be explored further.
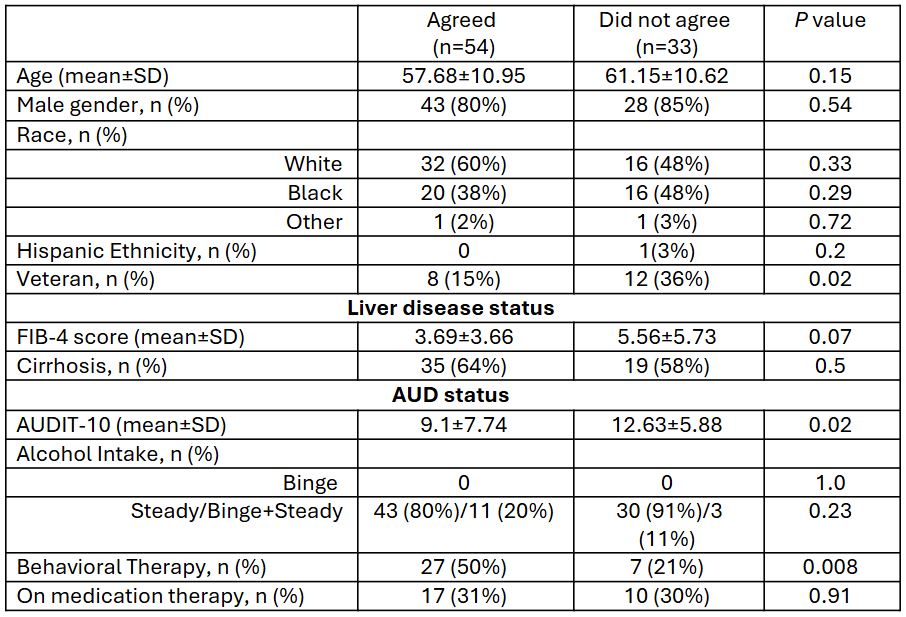
Figure: Table 1: Demographic and clinical characteristics of patients who agreed to participate and those who did not in a trial for FMT capsules in AUD+ALD
Disclosures:
Anas Aljabi indicated no relevant financial relationships.
Jasmohan Bajaj: Bausch – Grant/Research Support. Genfit – Grant/Research Support. Salix Pharmaceuticals – Clinical trial investigator. Sequana – Grant/Research Support.
Vinay Jahagirdar indicated no relevant financial relationships.
Amy Bartels indicated no relevant financial relationships.
Andrew Fagan indicated no relevant financial relationships.
Albert Arias indicated no relevant financial relationships.
Alexander Khoruts indicated no relevant financial relationships.
Anas Aljabi, MD1, Jasmohan S.. Bajaj, MD, MS, FACG2, Vinay Jahagirdar, MBBS3, Amy Bartels, RN1, Andrew Fagan, BS4, Albert Arias, MD, MS1, Alexander Khoruts, MD5. P3796 - Growing Acceptance of Live Microbial Therapeutics in Patients With Alcohol-Related Liver Disease to Reduce At-Risk Drinking, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Virginia Commonwealth University Health System, Richmond, VA; 2Virginia Commonwealth University; Central Virginia Veterans Healthcare System, Richmond, VA; 3Division of Gastroenterology, Hepatology and Nutrition, Virginia Commonwealth University Health System, Richmond, VA; 4Hunter Holmes McGuire VA Medical Center, Richmond, VA; 5University of Minnesota Medical Center, Minneapolis, MN
Introduction: Alcohol use disorder (AUD) and resultant liver disease (ALD) have a strong gut-liver-brain axis foundation. Recent animal and small human studies have shown that fecal microbiota transplant (FMT) can reduce problem drinking, but the acceptance of this modality in a larger group is unclear.
Aim: Determine acceptance of FMT in patients with concomitant AUD+ALD.
Methods: The Intestinal Microbiota Transplant in Alcohol-Associated Liver Disease (IMPACT) trial is studying whether capsule FMT versus placebo can enhance alcohol abstinence over 7 months in AUD+ALD patients. Recruitment is from a University and a VA hospital. Eligible subjects are age >21 yr, current alcohol use and AUD (AUDIT-10>8) with prior or current AUD therapies, ALD (FIB-4 >2.67 through cirrhosis) and able to provide stool samples. While main exclusions were food allergies, psychosis, allergies, concomitant drug use, dysphagia, hepatic encephalopathy (HE), or inability to consent. Reasons for acceptance or refusal to participate in the trial, specifically refusal due to FMT as a modality, were recorded. Comparisons between those who accepted versus declined were compared.
Results:
We evaluated 176 medical charts, of which 89 a priori did not meet the criteria (37 without ALD, 26 abstinent, 11 HE, 10 drug use, 6 other end-organ failures). The remaining 87 patients who qualified for the study were contacted, of whom 54 (62%) agreed to participate. Of the 33 who declined, 26 refused due to logistics and multiple study visits while only 7 patients refused due to discomfort with ingesting donor microbes.
Comparisons between those agreed or declined: As shown in Table 1, those who declined were more likely to be Veterans and have a higher AUDIT-10 and less likely to be on behavioral therapy than those agreed to participate. Demographics, proportion with cirrhosis, type of alcohol misuse pattern, and medication therapy for alcohol were statistically similar.
Declined due to FMT: was only seen in 8% (7 of 87) patients. All were men with cirrhosis of which 6 were Veterans. Three were on medical AUD therapy.
Discussion: In recruitment for a trial for FMT capsules in AUD+ALD who did not respond to behavioral or medical AUD therapy, most subjects approached were agreeable to participate. Most reasons for declining were related to logistics and only 8% of patients approached took issue with donor microbe ingestion. Live microbial biotherapeutic are acceptable to most AUD and ALD patients and if effective could be explored further.

Figure: Table 1: Demographic and clinical characteristics of patients who agreed to participate and those who did not in a trial for FMT capsules in AUD+ALD
Disclosures:
Anas Aljabi indicated no relevant financial relationships.
Jasmohan Bajaj: Bausch – Grant/Research Support. Genfit – Grant/Research Support. Salix Pharmaceuticals – Clinical trial investigator. Sequana – Grant/Research Support.
Vinay Jahagirdar indicated no relevant financial relationships.
Amy Bartels indicated no relevant financial relationships.
Andrew Fagan indicated no relevant financial relationships.
Albert Arias indicated no relevant financial relationships.
Alexander Khoruts indicated no relevant financial relationships.
Anas Aljabi, MD1, Jasmohan S.. Bajaj, MD, MS, FACG2, Vinay Jahagirdar, MBBS3, Amy Bartels, RN1, Andrew Fagan, BS4, Albert Arias, MD, MS1, Alexander Khoruts, MD5. P3796 - Growing Acceptance of Live Microbial Therapeutics in Patients With Alcohol-Related Liver Disease to Reduce At-Risk Drinking, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
