Monday Poster Session
Category: Liver
P3763 - Real-world Clinical Experience With Seladelpar in Primary Biliary Cholangitis
Monday, October 27, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- SK
Selena Kuo, MD
University of California San Diego Health
San Diego, CA
Presenting Author(s)
Selena Kuo, MD, Jeffrey Yin, PharmD, Rohit Loomba, MD
University of California San Diego Health, San Diego, CA
Introduction: Ursodeoxycholic acid is the only first-line therapy for primary biliary cholangitis (PBC). However, up to 40% of patients do not achieve an adequate biochemical response with ursodeoxycholic acid, which increases risk of disease progression and liver-related death. Seladelpar, a peroxisome proliferator-activated receptor delta agonist, recently received approval for treatment of PBC but there is limited knowledge regarding its efficacy in real-world clinical practice.
Methods: This is a longitudinal study of a cohort of adults with PBC who were started on seladelpar as second-line therapy. The primary end point evaluated was biochemical response and the secondary endpoint was normalization of the alkaline phosphatase level.
Results: Of the 18 patients, 11 were trialed on a second-line therapy prior to switching to seladelpar, whereas 7 of the patients started seladelpar as initial second-line therapy. Biochemical response was achieved in 56.2% of patients and normalization of alkaline phosphatase levels in 31.3% of patients after one month of treatment.
Discussion: In this cohort study of PBC patients, with a subset of patients who have been exposed to prior second-line therapy, we demonstrate that seladelpar elicited biochemical responses in real-world clinical practice.
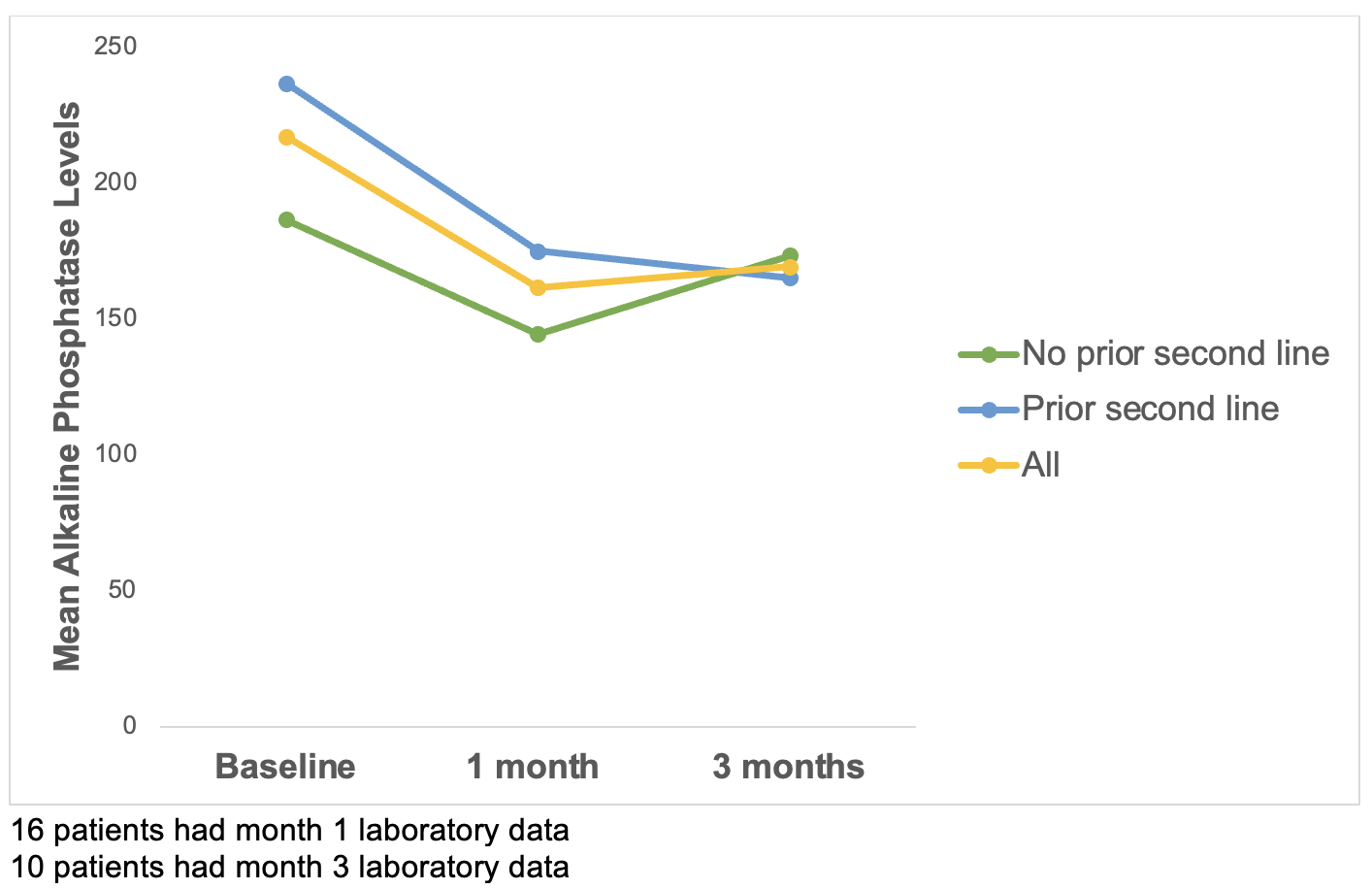
Figure: Table 1. Baseline Demographics
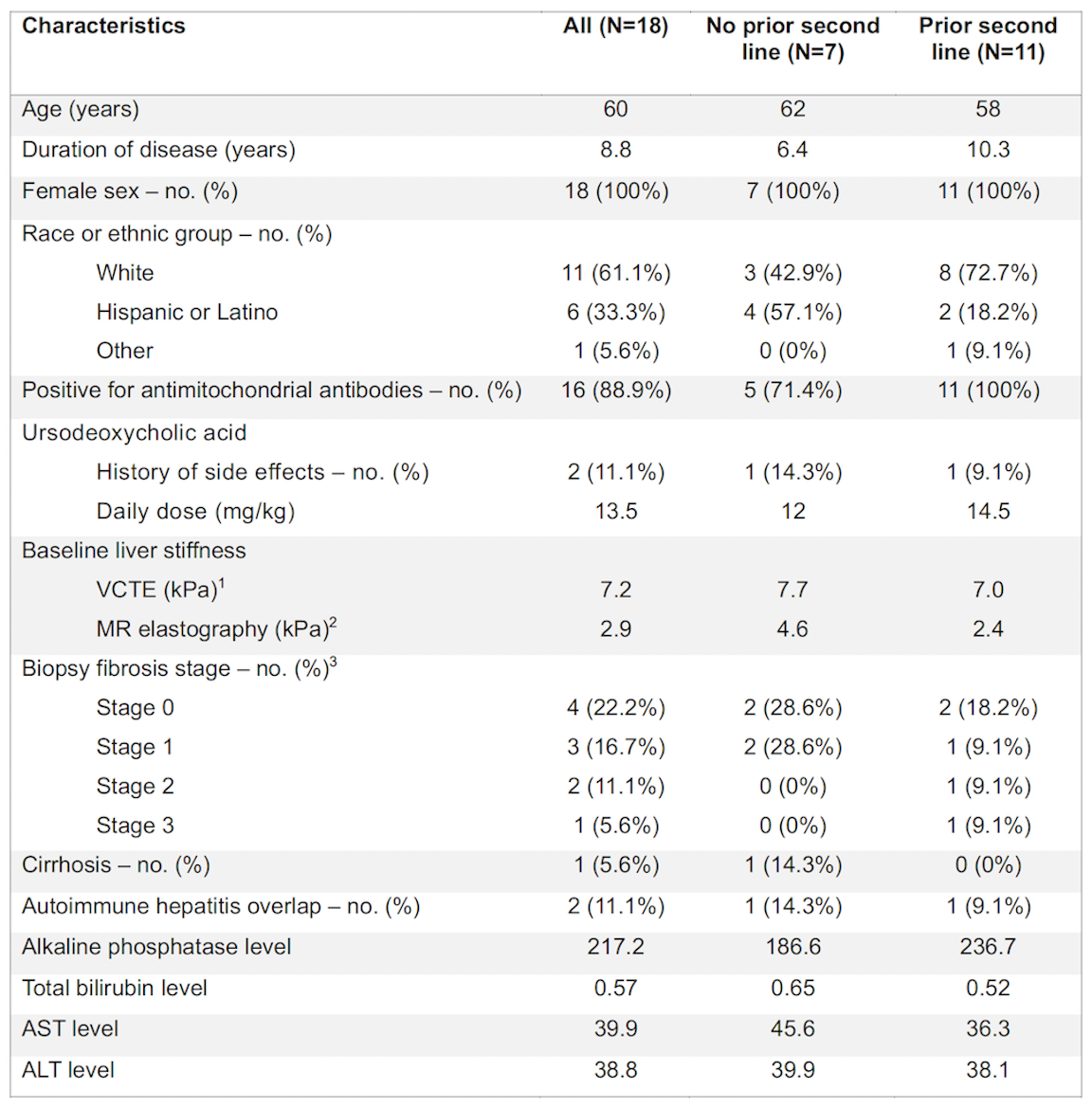
Figure: Figure 1. Alkaline phosphatase levels up to 3 months after treatment with seladalpar
Disclosures:
Selena Kuo indicated no relevant financial relationships.
Jeffrey Yin indicated no relevant financial relationships.
Rohit Loomba: 89 bio – Consultant. Aardvark Therapeutics – Consultant. Altimmune – Consultant. Arrowhead Pharmaceuticals – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. Boehringer-Ingelheim – Grant/Research Support. Bristol-Myers Squibb – Grant/Research Support. Cascade Pharmaceuticals – Consultant. Eli Lilly – Consultant, Grant/Research Support. Galectin Therapeutics – Grant/Research Support. Gilead – Consultant, Grant/Research Support. Glympse bio – Consultant. Hanmi – Grant/Research Support. Inipharma – Consultant. Intercept – Consultant, Grant/Research Support. Inventiva – Consultant, Grant/Research Support. Ionis – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Lipidio – Consultant. LipoNexus Inc. – Co-founder. Madrigal – Consultant, Grant/Research Support. Merck – Consultant, Grant/Research Support. Neurobo – Consultant. Novo Nordisk – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Sagimet biosciences – Consultant, Stock Options. sonic Incytes – Grant/Research Support. Takeda – Consultant. Terns Pharmaceuticals – Consultant, Grant/Research Support. Viking Therapeutics – Consultant.
Selena Kuo, MD, Jeffrey Yin, PharmD, Rohit Loomba, MD. P3763 - Real-world Clinical Experience With Seladelpar in Primary Biliary Cholangitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of California San Diego Health, San Diego, CA
Introduction: Ursodeoxycholic acid is the only first-line therapy for primary biliary cholangitis (PBC). However, up to 40% of patients do not achieve an adequate biochemical response with ursodeoxycholic acid, which increases risk of disease progression and liver-related death. Seladelpar, a peroxisome proliferator-activated receptor delta agonist, recently received approval for treatment of PBC but there is limited knowledge regarding its efficacy in real-world clinical practice.
Methods: This is a longitudinal study of a cohort of adults with PBC who were started on seladelpar as second-line therapy. The primary end point evaluated was biochemical response and the secondary endpoint was normalization of the alkaline phosphatase level.
Results: Of the 18 patients, 11 were trialed on a second-line therapy prior to switching to seladelpar, whereas 7 of the patients started seladelpar as initial second-line therapy. Biochemical response was achieved in 56.2% of patients and normalization of alkaline phosphatase levels in 31.3% of patients after one month of treatment.
Discussion: In this cohort study of PBC patients, with a subset of patients who have been exposed to prior second-line therapy, we demonstrate that seladelpar elicited biochemical responses in real-world clinical practice.

Figure: Table 1. Baseline Demographics

Figure: Figure 1. Alkaline phosphatase levels up to 3 months after treatment with seladalpar
Disclosures:
Selena Kuo indicated no relevant financial relationships.
Jeffrey Yin indicated no relevant financial relationships.
Rohit Loomba: 89 bio – Consultant. Aardvark Therapeutics – Consultant. Altimmune – Consultant. Arrowhead Pharmaceuticals – Consultant, Grant/Research Support. AstraZeneca – Consultant, Grant/Research Support. Boehringer-Ingelheim – Grant/Research Support. Bristol-Myers Squibb – Grant/Research Support. Cascade Pharmaceuticals – Consultant. Eli Lilly – Consultant, Grant/Research Support. Galectin Therapeutics – Grant/Research Support. Gilead – Consultant, Grant/Research Support. Glympse bio – Consultant. Hanmi – Grant/Research Support. Inipharma – Consultant. Intercept – Consultant, Grant/Research Support. Inventiva – Consultant, Grant/Research Support. Ionis – Consultant, Grant/Research Support. Janssen – Consultant, Grant/Research Support. Lipidio – Consultant. LipoNexus Inc. – Co-founder. Madrigal – Consultant, Grant/Research Support. Merck – Consultant, Grant/Research Support. Neurobo – Consultant. Novo Nordisk – Consultant, Grant/Research Support. Pfizer – Consultant, Grant/Research Support. Sagimet biosciences – Consultant, Stock Options. sonic Incytes – Grant/Research Support. Takeda – Consultant. Terns Pharmaceuticals – Consultant, Grant/Research Support. Viking Therapeutics – Consultant.
Selena Kuo, MD, Jeffrey Yin, PharmD, Rohit Loomba, MD. P3763 - Real-world Clinical Experience With Seladelpar in Primary Biliary Cholangitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
