Tuesday Poster Session
Category: Esophagus
P4891 - Budesonide Yields Superior Clinical and Histologic Remission in Adult Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, M. Rafiqul Islam, 2, Sakshi Nighania, MBBS3, Venkata Dileep Kumar Veldi, MBBS4, Arun Kumar. Maloth, MBBS5, Saraakshi Adiraju, 6, Nikhil Patel Pokar, MBBS7, Deepika Sanapala, MBBS8, Chika Chilaka, MBBS9, Aanshi S. Kothari, MBBS10, Ansh Rakeshkumar. Patel, MBBS11, Malavika Vinod, MBBS12, Saketh S. Mandiga, MBBS13
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh, Dhaka, Dhaka, Bangladesh; 3Veer narmada south gujarat university - NAMO MERI institute, Silvassa, Dadra and Nagar Haveli, India; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 6Osmania Medical College, Hyderabad, Telangana, India; 7Malla Reddy Institute of Medical Sciences, Hyderabad, Telangana, India; 8Katuri medical college, Gudivada, Andhra Pradesh, India; 9RWJBarnabas Health, Hamilton, NJ; 10Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India; 11GMERS Medical College and Hospital Himmatnagar, Gandhinagar, Gujarat, India; 12Baby memorial kannur, Mangalore, Karnataka, India; 13Osmania General Hospital and Medical College, Hyderabad, Telangana, India
Introduction: Eosinophilic esophagitis (EoE) affects 1:1,000 adults and is a leading cause of refractory dysphagia with progressive fibro-stenotic remodelling. Topical budesonide delivered as oro-dispersible tablets (ODT) or viscous suspensions is recommended, yet dose, formulation, and depth-of-remission data remain fragmented. To our knowledge no prior meta-analysis has integrated every placebo-controlled randomized controlled trial (RCTs) with formulation- and dose-level subgrouping, leaving clinicians without quantitative guidance. We pooled seven RCTs to quantify clinical, histologic, and deep remission and to explore dose-response.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing Budesonide with placebo or other formulations for EoE through May 2025. Data were analysed using R(version 4.4.3). Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Among seven RCTs with over 500 patients, budesonide 0.5–2 mg bid decisively out-performed placebo. Pooled RRs (random-effects): histologic remission 6.15 (1.41–26.8); partial histologic remission 13.18 (6.53–26.6); deep histologic remission 17.63 (3.73–83.4); clinical remission (dysphagia/odynophagia NRS ≤ 2) 15.45 (1.98–120.8); and failure to achieve histologic remission 0.22 (0.12–0.42). Benefits were greatest with 2 mg bid. Heterogeneity was low–moderate for partial remission but higher for deep and clinical endpoints.
Discussion: This meta-analysis shows that Budesonide increased deep histologic remission 18-fold and clinical remission 15-fold while halving non-responders. Benefits persisted across doses, though heterogeneity was high for deep endpoints and long-term effects remains unknown. By consolidating high-quality evidence for oral budesonide, these findings address a critical need and guide standardized topical therapy recommendations. Future pragmatic trials should evaluate maintenance strategies and sustained fibro-stenotic reversal.
Figure: Figures Showing Forest Plots of Histologic Remission with Overall and Dose Subgroups, Failure to Achieve Histologic Remission with Overall and Dose Subgroups, Deep Histologic Remission with Overall and Dose Subgroups, Clinical Remission - Dysphagia and Odynophagia NRS Score Less or Equal 2, Partial Histologic Remission with Overall and Dose Subgroups
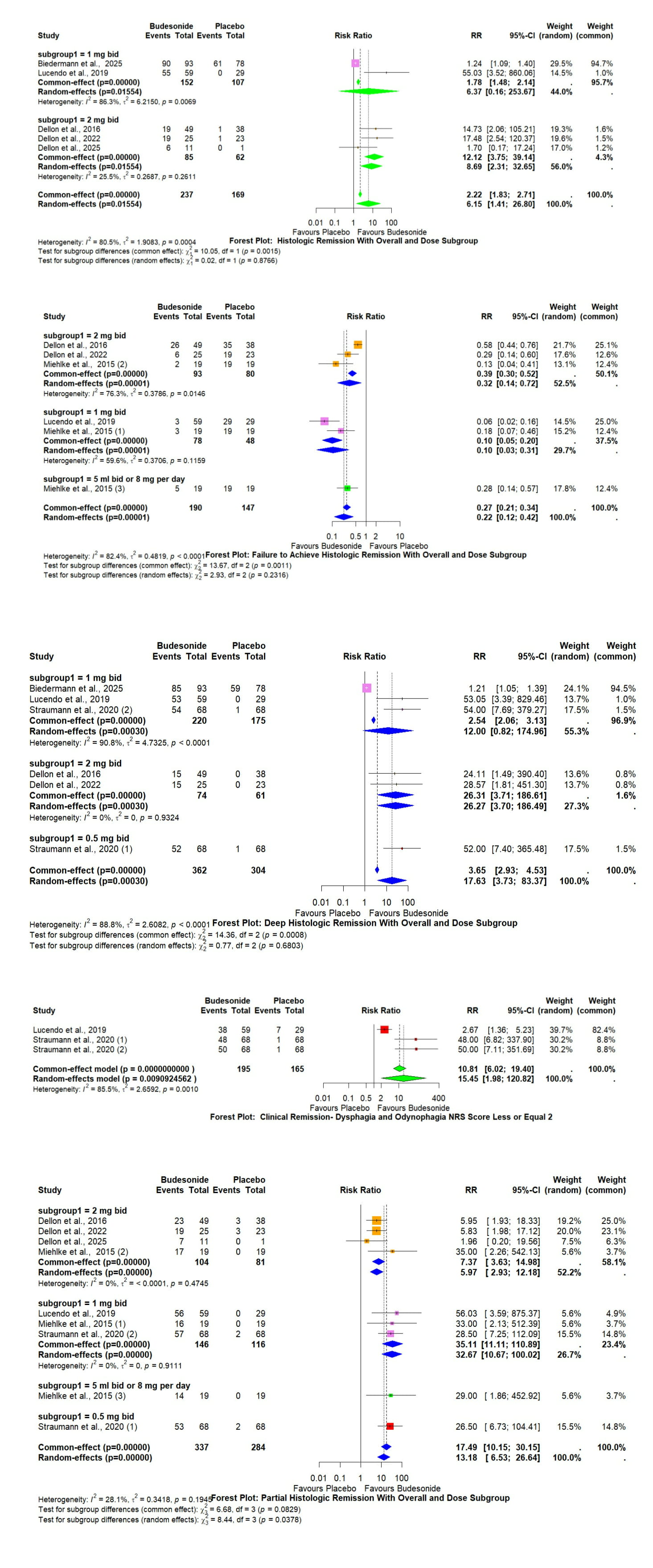
Figure: Prisma Flowchart
Disclosures:
Ashesh Das indicated no relevant financial relationships.
M. Rafiqul Islam indicated no relevant financial relationships.
Sakshi Nighania indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Saraakshi Adiraju indicated no relevant financial relationships.
Nikhil Patel Pokar indicated no relevant financial relationships.
Deepika Sanapala indicated no relevant financial relationships.
Chika Chilaka indicated no relevant financial relationships.
Aanshi Kothari indicated no relevant financial relationships.
Ansh Patel indicated no relevant financial relationships.
Malavika Vinod indicated no relevant financial relationships.
Saketh S. Mandiga indicated no relevant financial relationships.
Ashesh Das, MBBS1, M. Rafiqul Islam, 2, Sakshi Nighania, MBBS3, Venkata Dileep Kumar Veldi, MBBS4, Arun Kumar. Maloth, MBBS5, Saraakshi Adiraju, 6, Nikhil Patel Pokar, MBBS7, Deepika Sanapala, MBBS8, Chika Chilaka, MBBS9, Aanshi S. Kothari, MBBS10, Ansh Rakeshkumar. Patel, MBBS11, Malavika Vinod, MBBS12, Saketh S. Mandiga, MBBS13. P4891 - Budesonide Yields Superior Clinical and Histologic Remission in Adult Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Shaheed Suhrawardy Medical College and Hospital, Dhaka, Bangladesh, Dhaka, Dhaka, Bangladesh; 3Veer narmada south gujarat university - NAMO MERI institute, Silvassa, Dadra and Nagar Haveli, India; 4Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 5Kakatiya Medical College, Warangal, India, Warangal, Telangana, India; 6Osmania Medical College, Hyderabad, Telangana, India; 7Malla Reddy Institute of Medical Sciences, Hyderabad, Telangana, India; 8Katuri medical college, Gudivada, Andhra Pradesh, India; 9RWJBarnabas Health, Hamilton, NJ; 10Smt. NHL Municipal Medical College, Ahmedabad, Gujarat, India; 11GMERS Medical College and Hospital Himmatnagar, Gandhinagar, Gujarat, India; 12Baby memorial kannur, Mangalore, Karnataka, India; 13Osmania General Hospital and Medical College, Hyderabad, Telangana, India
Introduction: Eosinophilic esophagitis (EoE) affects 1:1,000 adults and is a leading cause of refractory dysphagia with progressive fibro-stenotic remodelling. Topical budesonide delivered as oro-dispersible tablets (ODT) or viscous suspensions is recommended, yet dose, formulation, and depth-of-remission data remain fragmented. To our knowledge no prior meta-analysis has integrated every placebo-controlled randomized controlled trial (RCTs) with formulation- and dose-level subgrouping, leaving clinicians without quantitative guidance. We pooled seven RCTs to quantify clinical, histologic, and deep remission and to explore dose-response.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing Budesonide with placebo or other formulations for EoE through May 2025. Data were analysed using R(version 4.4.3). Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Among seven RCTs with over 500 patients, budesonide 0.5–2 mg bid decisively out-performed placebo. Pooled RRs (random-effects): histologic remission 6.15 (1.41–26.8); partial histologic remission 13.18 (6.53–26.6); deep histologic remission 17.63 (3.73–83.4); clinical remission (dysphagia/odynophagia NRS ≤ 2) 15.45 (1.98–120.8); and failure to achieve histologic remission 0.22 (0.12–0.42). Benefits were greatest with 2 mg bid. Heterogeneity was low–moderate for partial remission but higher for deep and clinical endpoints.
Discussion: This meta-analysis shows that Budesonide increased deep histologic remission 18-fold and clinical remission 15-fold while halving non-responders. Benefits persisted across doses, though heterogeneity was high for deep endpoints and long-term effects remains unknown. By consolidating high-quality evidence for oral budesonide, these findings address a critical need and guide standardized topical therapy recommendations. Future pragmatic trials should evaluate maintenance strategies and sustained fibro-stenotic reversal.

Figure: Figures Showing Forest Plots of Histologic Remission with Overall and Dose Subgroups, Failure to Achieve Histologic Remission with Overall and Dose Subgroups, Deep Histologic Remission with Overall and Dose Subgroups, Clinical Remission - Dysphagia and Odynophagia NRS Score Less or Equal 2, Partial Histologic Remission with Overall and Dose Subgroups

Figure: Prisma Flowchart
Disclosures:
Ashesh Das indicated no relevant financial relationships.
M. Rafiqul Islam indicated no relevant financial relationships.
Sakshi Nighania indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Arun Maloth indicated no relevant financial relationships.
Saraakshi Adiraju indicated no relevant financial relationships.
Nikhil Patel Pokar indicated no relevant financial relationships.
Deepika Sanapala indicated no relevant financial relationships.
Chika Chilaka indicated no relevant financial relationships.
Aanshi Kothari indicated no relevant financial relationships.
Ansh Patel indicated no relevant financial relationships.
Malavika Vinod indicated no relevant financial relationships.
Saketh S. Mandiga indicated no relevant financial relationships.
Ashesh Das, MBBS1, M. Rafiqul Islam, 2, Sakshi Nighania, MBBS3, Venkata Dileep Kumar Veldi, MBBS4, Arun Kumar. Maloth, MBBS5, Saraakshi Adiraju, 6, Nikhil Patel Pokar, MBBS7, Deepika Sanapala, MBBS8, Chika Chilaka, MBBS9, Aanshi S. Kothari, MBBS10, Ansh Rakeshkumar. Patel, MBBS11, Malavika Vinod, MBBS12, Saketh S. Mandiga, MBBS13. P4891 - Budesonide Yields Superior Clinical and Histologic Remission in Adult Eosinophilic Esophagitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
