Tuesday Poster Session
Category: Esophagus
P4888 - Feasibility of Non-Operative Management Following Neoadjuvant Immune Checkpoint Inhibitors in Resectable dMMR/MSI-H Gastroesophageal Adenocarcinoma
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- FJ
Fares Jamal, MD (he/him/his)
Mayo Clinic
Phoenix, AZ
Presenting Author(s)
Fares Jamal, MD1, Oudai Sahwan, MBBS1, Cody Eslinger, MD, MS1, Shaylene McCue, BA2, Mitesh Borad, MD1, Mojun Zhu, MD2, Priya Pai, MBBS2, Hao Xie, MD2, Robert McWilliams, MD2, Nguyen H. Tran, MD2, Travis Grotz, MD2, Fang-Shu Ou, PhD2, Nabil Wasif, MD, MPH1, Jason S. Starr, MD3, Tanios Bekaii-Saab, MD1, Christina Wu, MBBS1, Harry Yoon, MD2, Daniel Ahn, DO1, Mohamad Bassam Sonbol, MD1
1Mayo Clinic, Phoenix, AZ; 2Mayo Clinic, Rochester, MN; 3Mayo Clinic, Jacksonville, FL
Introduction: Neoadjuvant immune checkpoint inhibitors (nICIs) have demonstrated high response rates in patients with deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H) gastroesophageal adenocarcinoma (GEA). The NEONIPIGA and INFINITY trials reported promising rates of pathological complete response (pCR), with early data suggesting non-operative management (NOM) may be feasible. We evaluated real-world outcomes of nICIs in resectable dMMR/MSI-H GEA, with emphasis on the potential for NOM.
Methods: We conducted a retrospective cohort study across multiple tertiary centers, identifying patients with histologically confirmed, resectable dMMR/MSI-H GEA treated with nICIs between April 1, 2017, and February 28, 2025. Patients with metastatic disease or incomplete records were excluded. Patients were treated with nICIs, followed either by surgery or NOM. Clinical complete response (cCR) was defined as absence of residual tumor on imaging and endoscopic evaluation. Primary outcomes included cCR, pathological complete response (pCR), and metastasis-free survival (MFS). Secondary outcomes included radiological complete response (rCR), and immune-related adverse events (irAEs).
Results: A total of 26 patients were included (median age 70; 61.5% male). Eight patients (30.8%) underwent surgical resection after nICIs, with 5 of 8 (62.5%) achieving pCR. Eighteen patients (69.2%) underwent or are undergoing NOM. Among 18 evaluable NOM patients, 10 of 14 evaluable patients (71.4%) achieved cCR, and 12 of 18 (66.7%) achieved rCR. 17 out of the 18 patients in the non-operative management cohort (94.4%) are alive and metastasis-free, with a median metastasis-free survival of 13.5 months (range: 4.1–81.8). Immune-related adverse events occurred in nine patients (34.6%), with no grade ≥3 toxicities.
Discussion: This study highlights the potential of nICIs to induce high response rates in resectable dMMR/MSI-H GEA, with many patients achieving durable clinical complete responses without surgery. These findings support the feasibility of a non-operative, organ-preserving approach in selected patients and warrant further investigation in prospective studies.
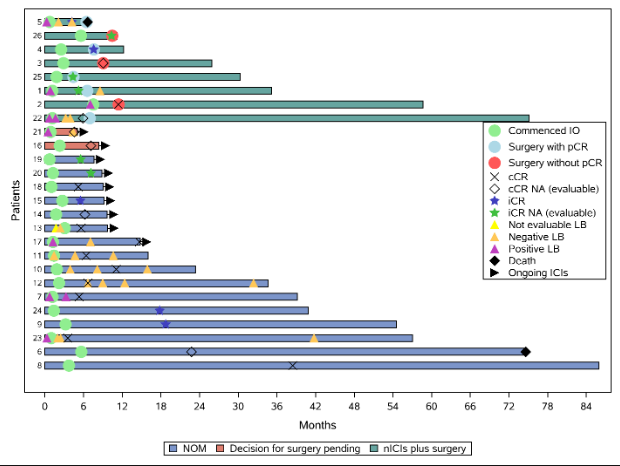
Figure: Swimmer plot graph illustrating management milestones for each patient. Time 0 = time of diagnosis.
IO: immunotherapy, pCR: pathological complete response, cCR: clinical complete response, NA: not achieved, iCR: imaging complete response, RD: residual disease, LB: liquid biopsy, nICIs: neoadjuvant immune checkpoint inhibitors, NOM: non-operative management.
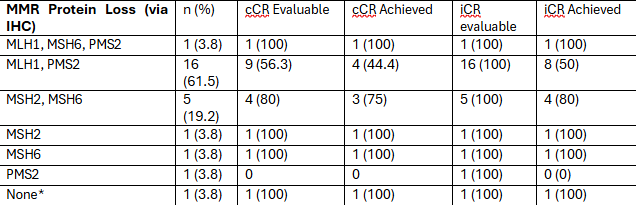
Figure: Efficacy Outcome by MMR Protein Loss
MMR: Mismatch Repair, cCR: clinical complete response, iCR: imaging complete response. Notes: a: Lynch syndrome patient (confirmed MSI-H via NGS)
Disclosures:
Fares Jamal indicated no relevant financial relationships.
Oudai Sahwan indicated no relevant financial relationships.
Cody Eslinger indicated no relevant financial relationships.
Shaylene McCue indicated no relevant financial relationships.
Mitesh Borad indicated no relevant financial relationships.
Mojun Zhu indicated no relevant financial relationships.
Priya Pai indicated no relevant financial relationships.
Hao Xie indicated no relevant financial relationships.
Robert McWilliams indicated no relevant financial relationships.
Nguyen Tran: AstraZeneca – Advisory Committee/Board Member, Consultant, Grant/Research Support. Dava Oncology – Speakers Bureau. EXACT SCIENCES – Grant/Research Support. Exelixis – Advisory Committee/Board Member. Genentech – Grant/Research Support. ipsen – Advisory Committee/Board Member.
Travis Grotz: Agenus – Consultant. Ferranova – Consultant. Intuitive – Grant/Research Support.
Fang-Shu Ou indicated no relevant financial relationships.
Nabil Wasif indicated no relevant financial relationships.
Jason S. Starr: Amgen – Grant/Research Support. Arcus Biosceince – Grant/Research Support. Boehringer-Ingelheim – Consultant, Grant/Research Support. Camurus Therapeutics – Grant/Research Support. Cardiff Oncology – Grant/Research Support. Exelixis – Consultant. Perspective Therapeutics – Grant/Research Support. RayezBio – Grant/Research Support.
Tanios Bekaii-Saab: 1Globe – Advisory Committee/Board Member. AbbVie – Consultant. Abgenomics – Grant/Research Support. Agios – Grant/Research Support. Arcus – Consultant, Grant/Research Support. Artiva and Sun Biopharma – Advisory Committee/Board Member. Arys – Grant/Research Support. AstraZeneca – Advisory Committee/Board Member, Consultant. Atreca – Grant/Research Support. Bayer – Consultant, Grant/Research Support. Beigene – Consultant. Blueprint Medicines – Consultant. BMS – Grant/Research Support. Boehringer Ingelheim – Consultant. Boston Biomedical – Grant/Research Support. Caladrius Biosciences – Consultant. Celgene – Grant/Research Support. Celularity – Consultant. Clovis – Grant/Research Support. Daiichi Sankyo – Consultant. Deciphera – Consultant. Eisai – Advisory Committee/Board Member, Consultant, Grant/Research Support. Elevar – Consultant. Exact Science – Consultant. Exelixis – Advisory Committee/Board Member, Consultant. Fibrogen – Advisory Committee/Board Member. Foundation Medicine and Sanofi – Consultant. Genentech – Consultant, Grant/Research Support. Glaxo – Consultant. Illumina – Consultant. Immuneering – Advisory Committee/Board Member. Imugene – Advisory Committee/Board Member, Intellectual Property/Patents. Incyte – Consultant, Grant/Research Support. Ipsen – Consultant, Grant/Research Support. Janssen – Consultant. Kanaph – Consultant. Lilly – Grant/Research Support. Merck – Advisory Committee/Board Member, Consultant. Merck KGaA – Consultant. Merus – Consultant, Grant/Research Support. Mirati – Grant/Research Support. Natera – Consultant. Novartis – Grant/Research Support. PanCan – Advisory Committee/Board Member. Pfizer – Consultant, Grant/Research Support. Recursion – Intellectual Property/Patents. Replimune – Advisory Committee/Board Member. Seattle Genetics – Consultant, Grant/Research Support. Servier – Consultant, Grant/Research Support. SmithKline – Consultant. Sobi – Consultant. Stemline – Consultant. Suzhou Kintor – Advisory Committee/Board Member. The Valley Hospital – Advisory Committee/Board Member. TreosBio – Consultant. Uptodate – Royalties. Xilio – Consultant. Xilis – Advisory Committee/Board Member. Zai Labs – Consultant.
Christina Wu: DoMore Diagnostics – Advisor or Review Panel Member. Exelixis – Advisor or Review Panel Member. GlaxkoSmithKline – Advisor or Review Panel Member. Merck – Advisor or Review Panel Member. Natera – Advisor or Review Panel Member. Pfizer – Grant/Research Support. Seagen – Grant/Research Support.
Harry Yoon: ALX Oncology – Consultant. Amgen – Consultant, Grant/Research Support. Aptitude Health, LLC – Consultant. ASCO – Travel Assistance. Astellas – travel/accommodation/expenses, Speakers Bureau. AstraZeneca – Consultant. Bayer Stivarga – Advisory Committee/Board Member. BeiGene – Consultant, Grant/Research Support, Travel/accommodation/expenses. Bristol Myer Squibb – Consultant. Daiichi Sankyo, Inc. – Advisory Committee/Board Member. Elevation Oncology – Travel/accommodation/expenses, Speakers Bureau. Eli Lilly And Company – Grant/Research Support. Estella's – Speakers Bureau. Genentech Inc – Grant/Research Support. Gilead Sciences Inc. - GI Cancer Council 2025 – Consultant. Global Learning Collaborative – Speakers Bureau. Jazz Pharmaceuticals GEA – Advisory Committee/Board Member. Macrogenics – Consultant. Medical Educator Consortium, Inc. – Speakers Bureau. Merck & Co., Inc – Consultant, Grant/Research Support. Merck Research Laboratories – Advisory Committee/Board Member. Merck Sharp & Dohme Corp. – Consultant. MJH Life Sciences – Expert Testimony, Speakers Bureau. National Cancer Institute – Grant/Research Support. NCCN-National Comprehensive Cancer Network – Travel Assistance, Speakers Bureau. Novartis – Consultant. OncLive – Advisor or Review Panel Member. OncXerna – Consultant. PRIME – Travel/accommodation/expenses, Speakers Bureau. Research to Practice – Speakers Bureau. Total Health – Speakers Bureau. Zymeworks – Consultant.
Daniel Ahn indicated no relevant financial relationships.
Mohamad Bassam Sonbol indicated no relevant financial relationships.
Fares Jamal, MD1, Oudai Sahwan, MBBS1, Cody Eslinger, MD, MS1, Shaylene McCue, BA2, Mitesh Borad, MD1, Mojun Zhu, MD2, Priya Pai, MBBS2, Hao Xie, MD2, Robert McWilliams, MD2, Nguyen H. Tran, MD2, Travis Grotz, MD2, Fang-Shu Ou, PhD2, Nabil Wasif, MD, MPH1, Jason S. Starr, MD3, Tanios Bekaii-Saab, MD1, Christina Wu, MBBS1, Harry Yoon, MD2, Daniel Ahn, DO1, Mohamad Bassam Sonbol, MD1. P4888 - Feasibility of Non-Operative Management Following Neoadjuvant Immune Checkpoint Inhibitors in Resectable dMMR/MSI-H Gastroesophageal Adenocarcinoma, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Phoenix, AZ; 2Mayo Clinic, Rochester, MN; 3Mayo Clinic, Jacksonville, FL
Introduction: Neoadjuvant immune checkpoint inhibitors (nICIs) have demonstrated high response rates in patients with deficient mismatch repair (dMMR)/microsatellite instability-high (MSI-H) gastroesophageal adenocarcinoma (GEA). The NEONIPIGA and INFINITY trials reported promising rates of pathological complete response (pCR), with early data suggesting non-operative management (NOM) may be feasible. We evaluated real-world outcomes of nICIs in resectable dMMR/MSI-H GEA, with emphasis on the potential for NOM.
Methods: We conducted a retrospective cohort study across multiple tertiary centers, identifying patients with histologically confirmed, resectable dMMR/MSI-H GEA treated with nICIs between April 1, 2017, and February 28, 2025. Patients with metastatic disease or incomplete records were excluded. Patients were treated with nICIs, followed either by surgery or NOM. Clinical complete response (cCR) was defined as absence of residual tumor on imaging and endoscopic evaluation. Primary outcomes included cCR, pathological complete response (pCR), and metastasis-free survival (MFS). Secondary outcomes included radiological complete response (rCR), and immune-related adverse events (irAEs).
Results: A total of 26 patients were included (median age 70; 61.5% male). Eight patients (30.8%) underwent surgical resection after nICIs, with 5 of 8 (62.5%) achieving pCR. Eighteen patients (69.2%) underwent or are undergoing NOM. Among 18 evaluable NOM patients, 10 of 14 evaluable patients (71.4%) achieved cCR, and 12 of 18 (66.7%) achieved rCR. 17 out of the 18 patients in the non-operative management cohort (94.4%) are alive and metastasis-free, with a median metastasis-free survival of 13.5 months (range: 4.1–81.8). Immune-related adverse events occurred in nine patients (34.6%), with no grade ≥3 toxicities.
Discussion: This study highlights the potential of nICIs to induce high response rates in resectable dMMR/MSI-H GEA, with many patients achieving durable clinical complete responses without surgery. These findings support the feasibility of a non-operative, organ-preserving approach in selected patients and warrant further investigation in prospective studies.

Figure: Swimmer plot graph illustrating management milestones for each patient. Time 0 = time of diagnosis.
IO: immunotherapy, pCR: pathological complete response, cCR: clinical complete response, NA: not achieved, iCR: imaging complete response, RD: residual disease, LB: liquid biopsy, nICIs: neoadjuvant immune checkpoint inhibitors, NOM: non-operative management.

Figure: Efficacy Outcome by MMR Protein Loss
MMR: Mismatch Repair, cCR: clinical complete response, iCR: imaging complete response. Notes: a: Lynch syndrome patient (confirmed MSI-H via NGS)
Disclosures:
Fares Jamal indicated no relevant financial relationships.
Oudai Sahwan indicated no relevant financial relationships.
Cody Eslinger indicated no relevant financial relationships.
Shaylene McCue indicated no relevant financial relationships.
Mitesh Borad indicated no relevant financial relationships.
Mojun Zhu indicated no relevant financial relationships.
Priya Pai indicated no relevant financial relationships.
Hao Xie indicated no relevant financial relationships.
Robert McWilliams indicated no relevant financial relationships.
Nguyen Tran: AstraZeneca – Advisory Committee/Board Member, Consultant, Grant/Research Support. Dava Oncology – Speakers Bureau. EXACT SCIENCES – Grant/Research Support. Exelixis – Advisory Committee/Board Member. Genentech – Grant/Research Support. ipsen – Advisory Committee/Board Member.
Travis Grotz: Agenus – Consultant. Ferranova – Consultant. Intuitive – Grant/Research Support.
Fang-Shu Ou indicated no relevant financial relationships.
Nabil Wasif indicated no relevant financial relationships.
Jason S. Starr: Amgen – Grant/Research Support. Arcus Biosceince – Grant/Research Support. Boehringer-Ingelheim – Consultant, Grant/Research Support. Camurus Therapeutics – Grant/Research Support. Cardiff Oncology – Grant/Research Support. Exelixis – Consultant. Perspective Therapeutics – Grant/Research Support. RayezBio – Grant/Research Support.
Tanios Bekaii-Saab: 1Globe – Advisory Committee/Board Member. AbbVie – Consultant. Abgenomics – Grant/Research Support. Agios – Grant/Research Support. Arcus – Consultant, Grant/Research Support. Artiva and Sun Biopharma – Advisory Committee/Board Member. Arys – Grant/Research Support. AstraZeneca – Advisory Committee/Board Member, Consultant. Atreca – Grant/Research Support. Bayer – Consultant, Grant/Research Support. Beigene – Consultant. Blueprint Medicines – Consultant. BMS – Grant/Research Support. Boehringer Ingelheim – Consultant. Boston Biomedical – Grant/Research Support. Caladrius Biosciences – Consultant. Celgene – Grant/Research Support. Celularity – Consultant. Clovis – Grant/Research Support. Daiichi Sankyo – Consultant. Deciphera – Consultant. Eisai – Advisory Committee/Board Member, Consultant, Grant/Research Support. Elevar – Consultant. Exact Science – Consultant. Exelixis – Advisory Committee/Board Member, Consultant. Fibrogen – Advisory Committee/Board Member. Foundation Medicine and Sanofi – Consultant. Genentech – Consultant, Grant/Research Support. Glaxo – Consultant. Illumina – Consultant. Immuneering – Advisory Committee/Board Member. Imugene – Advisory Committee/Board Member, Intellectual Property/Patents. Incyte – Consultant, Grant/Research Support. Ipsen – Consultant, Grant/Research Support. Janssen – Consultant. Kanaph – Consultant. Lilly – Grant/Research Support. Merck – Advisory Committee/Board Member, Consultant. Merck KGaA – Consultant. Merus – Consultant, Grant/Research Support. Mirati – Grant/Research Support. Natera – Consultant. Novartis – Grant/Research Support. PanCan – Advisory Committee/Board Member. Pfizer – Consultant, Grant/Research Support. Recursion – Intellectual Property/Patents. Replimune – Advisory Committee/Board Member. Seattle Genetics – Consultant, Grant/Research Support. Servier – Consultant, Grant/Research Support. SmithKline – Consultant. Sobi – Consultant. Stemline – Consultant. Suzhou Kintor – Advisory Committee/Board Member. The Valley Hospital – Advisory Committee/Board Member. TreosBio – Consultant. Uptodate – Royalties. Xilio – Consultant. Xilis – Advisory Committee/Board Member. Zai Labs – Consultant.
Christina Wu: DoMore Diagnostics – Advisor or Review Panel Member. Exelixis – Advisor or Review Panel Member. GlaxkoSmithKline – Advisor or Review Panel Member. Merck – Advisor or Review Panel Member. Natera – Advisor or Review Panel Member. Pfizer – Grant/Research Support. Seagen – Grant/Research Support.
Harry Yoon: ALX Oncology – Consultant. Amgen – Consultant, Grant/Research Support. Aptitude Health, LLC – Consultant. ASCO – Travel Assistance. Astellas – travel/accommodation/expenses, Speakers Bureau. AstraZeneca – Consultant. Bayer Stivarga – Advisory Committee/Board Member. BeiGene – Consultant, Grant/Research Support, Travel/accommodation/expenses. Bristol Myer Squibb – Consultant. Daiichi Sankyo, Inc. – Advisory Committee/Board Member. Elevation Oncology – Travel/accommodation/expenses, Speakers Bureau. Eli Lilly And Company – Grant/Research Support. Estella's – Speakers Bureau. Genentech Inc – Grant/Research Support. Gilead Sciences Inc. - GI Cancer Council 2025 – Consultant. Global Learning Collaborative – Speakers Bureau. Jazz Pharmaceuticals GEA – Advisory Committee/Board Member. Macrogenics – Consultant. Medical Educator Consortium, Inc. – Speakers Bureau. Merck & Co., Inc – Consultant, Grant/Research Support. Merck Research Laboratories – Advisory Committee/Board Member. Merck Sharp & Dohme Corp. – Consultant. MJH Life Sciences – Expert Testimony, Speakers Bureau. National Cancer Institute – Grant/Research Support. NCCN-National Comprehensive Cancer Network – Travel Assistance, Speakers Bureau. Novartis – Consultant. OncLive – Advisor or Review Panel Member. OncXerna – Consultant. PRIME – Travel/accommodation/expenses, Speakers Bureau. Research to Practice – Speakers Bureau. Total Health – Speakers Bureau. Zymeworks – Consultant.
Daniel Ahn indicated no relevant financial relationships.
Mohamad Bassam Sonbol indicated no relevant financial relationships.
Fares Jamal, MD1, Oudai Sahwan, MBBS1, Cody Eslinger, MD, MS1, Shaylene McCue, BA2, Mitesh Borad, MD1, Mojun Zhu, MD2, Priya Pai, MBBS2, Hao Xie, MD2, Robert McWilliams, MD2, Nguyen H. Tran, MD2, Travis Grotz, MD2, Fang-Shu Ou, PhD2, Nabil Wasif, MD, MPH1, Jason S. Starr, MD3, Tanios Bekaii-Saab, MD1, Christina Wu, MBBS1, Harry Yoon, MD2, Daniel Ahn, DO1, Mohamad Bassam Sonbol, MD1. P4888 - Feasibility of Non-Operative Management Following Neoadjuvant Immune Checkpoint Inhibitors in Resectable dMMR/MSI-H Gastroesophageal Adenocarcinoma, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
