Tuesday Poster Session
Category: Esophagus
P4886 - Adoption of Esophageal Impedance Planimetry After FDA Approval: A Retrospective Study of Utilization and Patient Characteristics
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Julton Tomanguillo Chumbe, MD
Charleston Area Medical Center
Charleston, WV
Presenting Author(s)
Julton Tomanguillo, MD, Anum Khakwani, MD, Nisar Amin, MD, Harleen Chela, MD, Ebubekir Daglilar, MD
Charleston Area Medical Center, Charleston, WV
Introduction: The endoluminal functional imaging probe (EndoFLIP) uses impedance planimetry to evaluate esophageal distensibility and esophagogastric junction dynamics, addressing limitations of traditional diagnostic methods. This study elucidates the utilization patterns of esophageal impedance planimetry since receiving FDA approval in 2017.
Methods: This descriptive study utilized data from the TriNetX platform to examine patients who underwent esophageal impedance planimetry (CPT-91040) between March 2017 and December 2024. All statistical analyses were conducted within the TriNetX platform. Descriptive statistics were employed and included frequencies, percentages, and mean ± standard deviation.
Results: During the eight-year study period, a total of 13,139 patients underwent esophageal impedance planimetry procedure following its FDA approval. The patient population ranged in age from 18 to 90 years, with a mean age of 61±17 years. Females were more frequently represented in the cohort than males (61.0% vs 35.9%). The majority of patients receiving esophageal impedance planimetry procedure were non-Hispanic/Latino (80.0%). The most prevalent races among the patients were White (68.86%) and Black/African American (11.1%), followed by Asian (3.5%), American Indian/Alaska Native (0.3%), and Native Hawaiian/Other Pacific Islander (0.1%). Among examined patients, 7,628 (58.05%) underwent esophageal impedance planimetry without prior esophageal manometry (EM), and of these, 1,113 (14.5%) subsequently underwent EM post esophageal impedance planimetry. Since its approval, the utilization of esophageal impedance planimetry has demonstrated an upward trend, with 246 esophageal impedance planimetry procedures performed in 2017, and increasing to 3,035 procedures in 2024 (Figure A). Lastly, a total of 1,309 patients diagnosed with Achalasia underwent esophageal impedance planimetry without manometry at any point in time; of these, 271(20.70%) underwent transoral lower esophageal myotomy, and 102 (7.80%) underwent esophagomyotomy Heller type surgical intervention.
Discussion: Since its approval by the FDA, esophageal impedance planimetry utilization has progressively increased since 2017. Overall females, non-Hispanic individuals, and White patients comprised the majority of the patients undergoing this procedure. Determining utilization trends of esophageal impedance planimetry is essential for understanding its evolving role in the diagnosis and management of esophageal dysmotility.
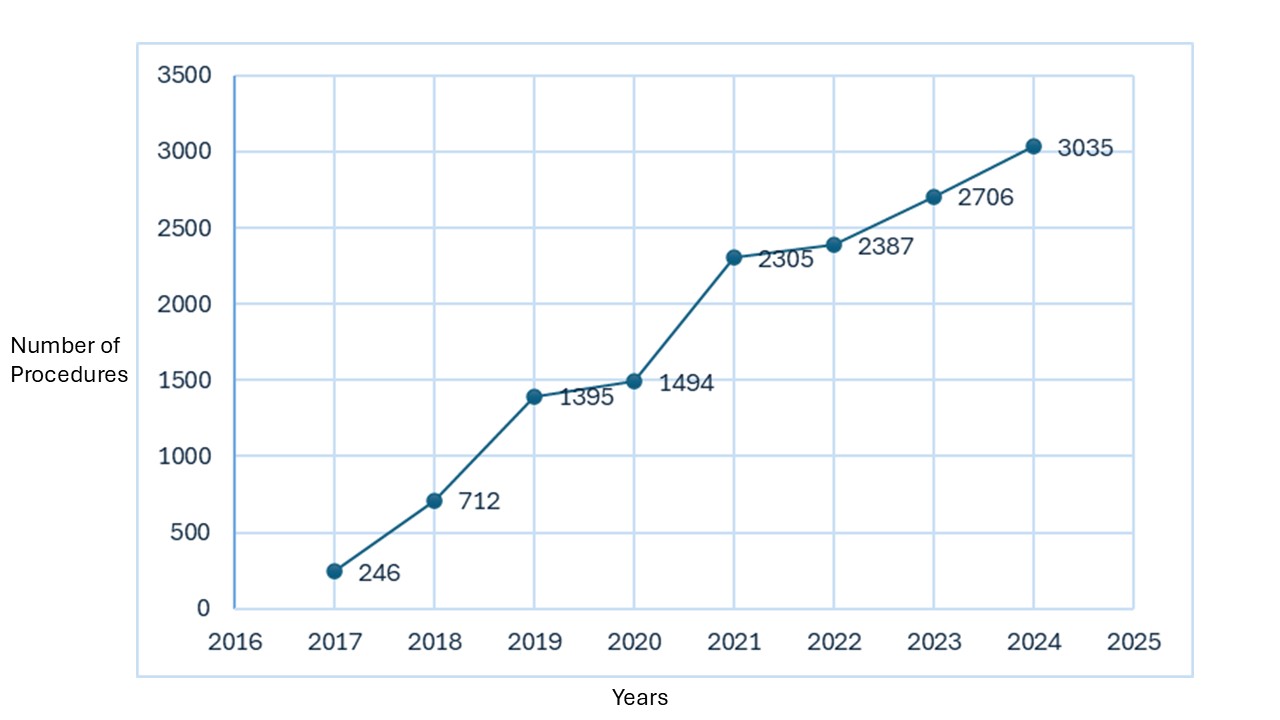
Figure: Figure A: Number of Esophageal Impedance Planimetry per Year
Disclosures:
Julton Tomanguillo indicated no relevant financial relationships.
Anum Khakwani indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Julton Tomanguillo, MD, Anum Khakwani, MD, Nisar Amin, MD, Harleen Chela, MD, Ebubekir Daglilar, MD. P4886 - Adoption of Esophageal Impedance Planimetry After FDA Approval: A Retrospective Study of Utilization and Patient Characteristics, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Charleston Area Medical Center, Charleston, WV
Introduction: The endoluminal functional imaging probe (EndoFLIP) uses impedance planimetry to evaluate esophageal distensibility and esophagogastric junction dynamics, addressing limitations of traditional diagnostic methods. This study elucidates the utilization patterns of esophageal impedance planimetry since receiving FDA approval in 2017.
Methods: This descriptive study utilized data from the TriNetX platform to examine patients who underwent esophageal impedance planimetry (CPT-91040) between March 2017 and December 2024. All statistical analyses were conducted within the TriNetX platform. Descriptive statistics were employed and included frequencies, percentages, and mean ± standard deviation.
Results: During the eight-year study period, a total of 13,139 patients underwent esophageal impedance planimetry procedure following its FDA approval. The patient population ranged in age from 18 to 90 years, with a mean age of 61±17 years. Females were more frequently represented in the cohort than males (61.0% vs 35.9%). The majority of patients receiving esophageal impedance planimetry procedure were non-Hispanic/Latino (80.0%). The most prevalent races among the patients were White (68.86%) and Black/African American (11.1%), followed by Asian (3.5%), American Indian/Alaska Native (0.3%), and Native Hawaiian/Other Pacific Islander (0.1%). Among examined patients, 7,628 (58.05%) underwent esophageal impedance planimetry without prior esophageal manometry (EM), and of these, 1,113 (14.5%) subsequently underwent EM post esophageal impedance planimetry. Since its approval, the utilization of esophageal impedance planimetry has demonstrated an upward trend, with 246 esophageal impedance planimetry procedures performed in 2017, and increasing to 3,035 procedures in 2024 (Figure A). Lastly, a total of 1,309 patients diagnosed with Achalasia underwent esophageal impedance planimetry without manometry at any point in time; of these, 271(20.70%) underwent transoral lower esophageal myotomy, and 102 (7.80%) underwent esophagomyotomy Heller type surgical intervention.
Discussion: Since its approval by the FDA, esophageal impedance planimetry utilization has progressively increased since 2017. Overall females, non-Hispanic individuals, and White patients comprised the majority of the patients undergoing this procedure. Determining utilization trends of esophageal impedance planimetry is essential for understanding its evolving role in the diagnosis and management of esophageal dysmotility.

Figure: Figure A: Number of Esophageal Impedance Planimetry per Year
Disclosures:
Julton Tomanguillo indicated no relevant financial relationships.
Anum Khakwani indicated no relevant financial relationships.
Nisar Amin indicated no relevant financial relationships.
Harleen Chela indicated no relevant financial relationships.
Ebubekir Daglilar indicated no relevant financial relationships.
Julton Tomanguillo, MD, Anum Khakwani, MD, Nisar Amin, MD, Harleen Chela, MD, Ebubekir Daglilar, MD. P4886 - Adoption of Esophageal Impedance Planimetry After FDA Approval: A Retrospective Study of Utilization and Patient Characteristics, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
