Tuesday Poster Session
Category: Functional Bowel Disease
P5110 - Sustained Efficacy and Tolerability of Vibrant Capsule Therapy in a 77-Year-Old Female With Chronic Constipation: A 24-Month Case Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Darren M. Brenner, MD, FACG (he/him/his)
Professor of Medicine and Surgery
Northwestern University Feinberg School of Medicine, Chicago, IL, US
Chicago, IL
Presenting Author(s)
Darren M.. Brenner, MD
Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL
Introduction: Chronic idiopathic constipation (CIC) is a common condition frequently complicated by intolerance to conventional therapies. This case study describes the long-term use of Vibrant, a novel non-pharmacological, vibration-based capsule therapy, in a patient with treatment-refractory CIC.
Case Description/
Methods: A 77-year-old female with a >5-year history of CIC presented with an average of 0–1 complete spontaneous bowel movements (CSBMs) per week prior to Vibrant therapy. Previous treatments included linaclotide, plecanatide, and magnesium-based products, which provided only partial relief and were associated with adverse effects including bloating and diarrhea. The patient reported:
“I had bloating and was not going to the bathroom without taking large amounts of magnesium citrate, had 2 BMs per week, and diarrhea.”
In February 2023, she initiated Vibrant capsule therapy. Initially, she used it in combination with Chinese herbal remedies, averaging 4.6 capsules per week. She also actively engaged with the Vibrant companion app (fig 1), noting:
“The reports have been a good way to help me track my progress.”
Discussion: 6-Month Follow-Up (November 2023): Patient reported 5 complete spontaneous bowel movements (CSBMs)/week and a shift from diarrhea with medications or hard stool without medications to normal stool consistency. She described her progress as satisfactory and attributed improvement to Vibrant and lifestyle changes.
24-Month Follow-Up (March 2025): Now using Vibrant as monotherapy, the patient maintained bowel regularity and noted further improvement:
“My constipation is non-existent now. It is also partially due to me changing the timing of my calcium vitamin intake.”
The patient reported:
This case demonstrates that Vibrant capsule therapy can be safely and effectively used for long-term management of CIC in older adults. The patient reported sustained symptom relief, reduced treatment burden, and high satisfaction over 24 months of use. Vibrant may offer a well-tolerated, non-pharmacologic alternative in elderly patients with refractory constipation.
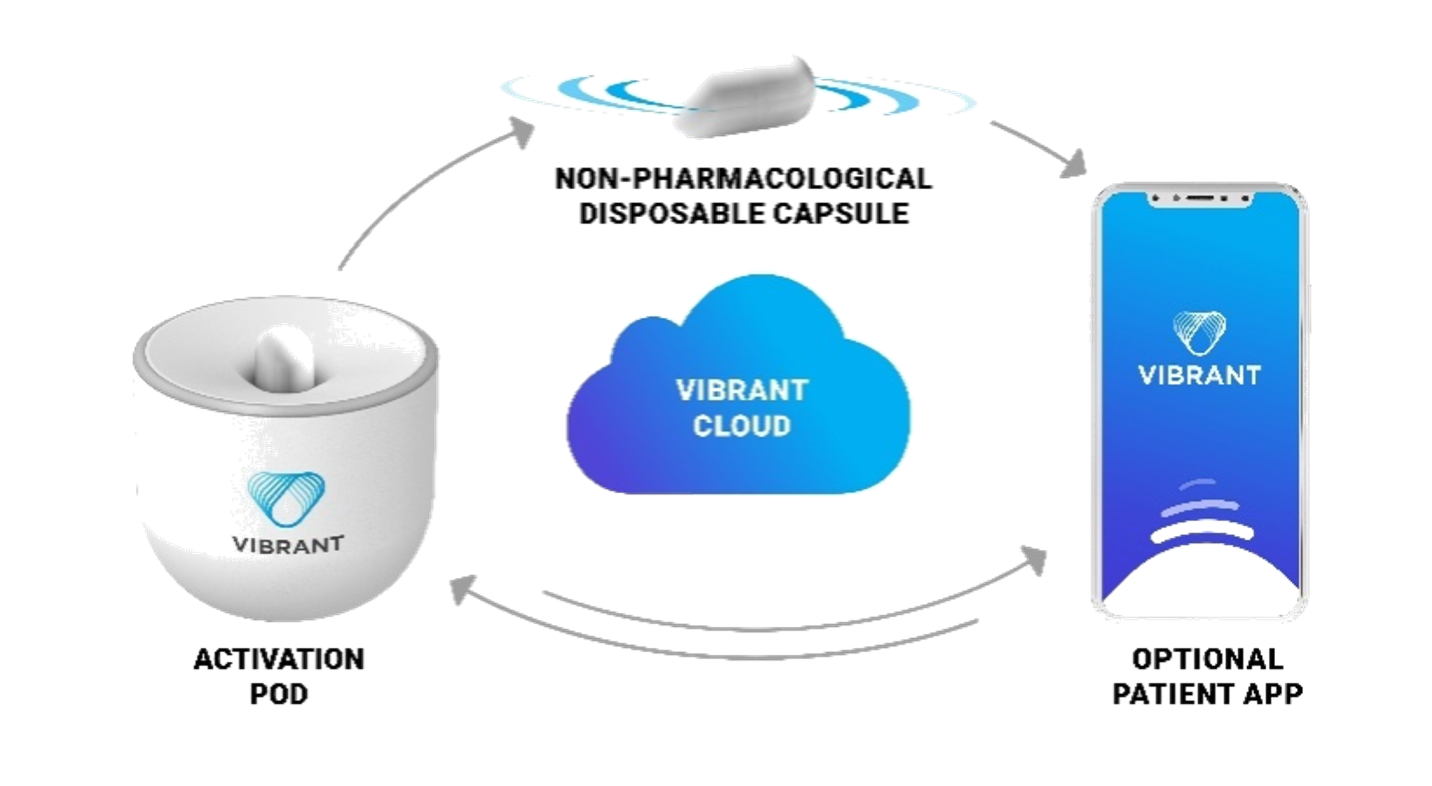
Figure: Patient Assessment (Table 1)
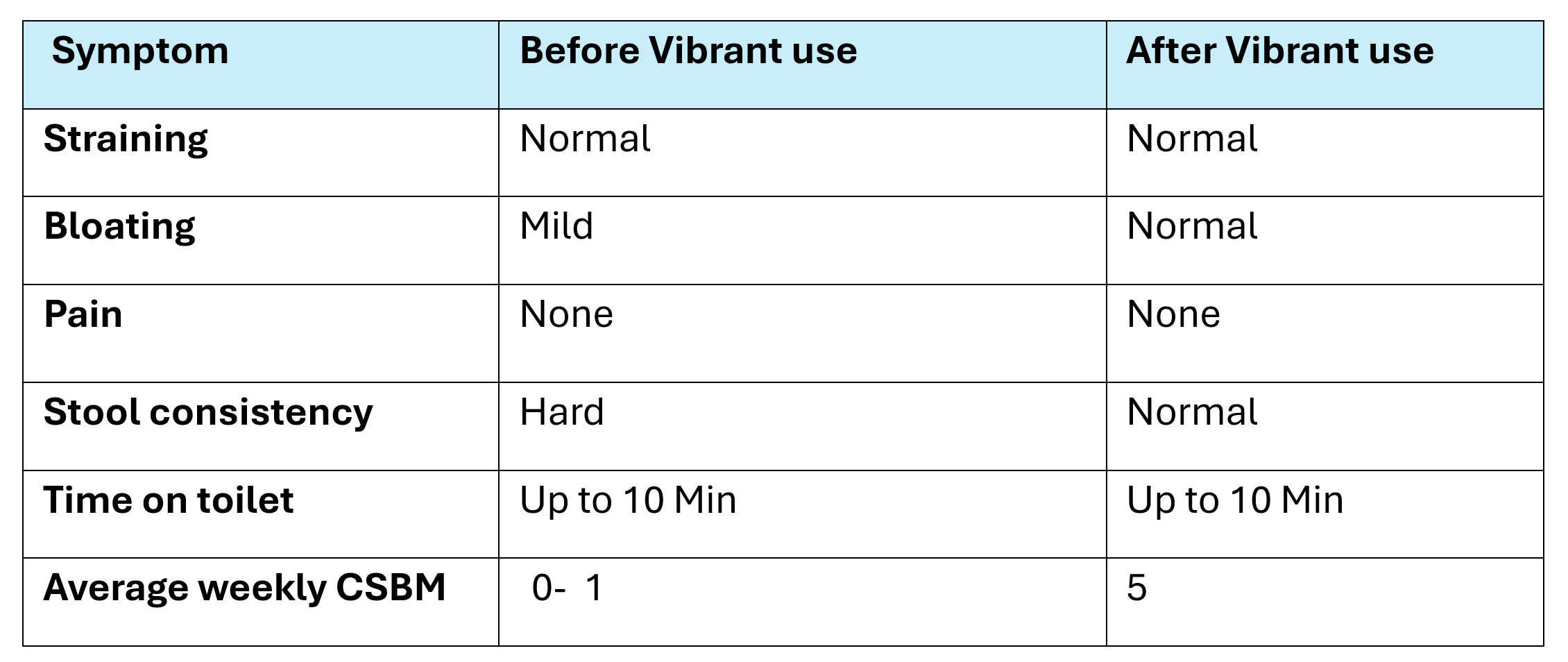
Figure: The Vibrant System (Fig 1)
Disclosures:
Darren Brenner: Alnylam Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Anji Pharma, Ardelyx – Advisor or Review Panel Member. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Bayer, Blueprint Medicines, CinPhloro Pharma, Dr Reddy’s Laboratories, Gemelli Biotech, Laborie – Consultant. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Salix Pharmaceuticals – Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speaker. International Foundation for GI Disorders – Advisory Committee/Board Member. Mahana Therapeutics, Owlstone Medical, Salix Pharmaceuticals, Vibrant Pharma – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant, Speaker.
Darren M.. Brenner, MD. P5110 - Sustained Efficacy and Tolerability of Vibrant Capsule Therapy in a 77-Year-Old Female With Chronic Constipation: A 24-Month Case Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Northwestern University Feinberg School of Medicine, Chicago, IL, US, Chicago, IL
Introduction: Chronic idiopathic constipation (CIC) is a common condition frequently complicated by intolerance to conventional therapies. This case study describes the long-term use of Vibrant, a novel non-pharmacological, vibration-based capsule therapy, in a patient with treatment-refractory CIC.
Case Description/
Methods: A 77-year-old female with a >5-year history of CIC presented with an average of 0–1 complete spontaneous bowel movements (CSBMs) per week prior to Vibrant therapy. Previous treatments included linaclotide, plecanatide, and magnesium-based products, which provided only partial relief and were associated with adverse effects including bloating and diarrhea. The patient reported:
“I had bloating and was not going to the bathroom without taking large amounts of magnesium citrate, had 2 BMs per week, and diarrhea.”
In February 2023, she initiated Vibrant capsule therapy. Initially, she used it in combination with Chinese herbal remedies, averaging 4.6 capsules per week. She also actively engaged with the Vibrant companion app (fig 1), noting:
“The reports have been a good way to help me track my progress.”
Discussion: 6-Month Follow-Up (November 2023): Patient reported 5 complete spontaneous bowel movements (CSBMs)/week and a shift from diarrhea with medications or hard stool without medications to normal stool consistency. She described her progress as satisfactory and attributed improvement to Vibrant and lifestyle changes.
24-Month Follow-Up (March 2025): Now using Vibrant as monotherapy, the patient maintained bowel regularity and noted further improvement:
“My constipation is non-existent now. It is also partially due to me changing the timing of my calcium vitamin intake.”
The patient reported:
- No adverse events with Vibrant.
- Decreased use of additional constipation therapies and healthcare resources.
- Temporary treatment interruption due to an MRI (April 2024), requiring X-rays and bowel prep for capsule clearance. She resumed Vibrant without issue.
This case demonstrates that Vibrant capsule therapy can be safely and effectively used for long-term management of CIC in older adults. The patient reported sustained symptom relief, reduced treatment burden, and high satisfaction over 24 months of use. Vibrant may offer a well-tolerated, non-pharmacologic alternative in elderly patients with refractory constipation.

Figure: Patient Assessment (Table 1)

Figure: The Vibrant System (Fig 1)
Disclosures:
Darren Brenner: Alnylam Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Anji Pharma, Ardelyx – Advisor or Review Panel Member. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Bayer, Blueprint Medicines, CinPhloro Pharma, Dr Reddy’s Laboratories, Gemelli Biotech, Laborie – Consultant. Ardelyx, AbbVie, Ironwood Pharmaceuticals, Salix Pharmaceuticals – Speakers Bureau. Entrinsic Bioscience – Advisor or Review Panel Member, Consultant, Speaker. International Foundation for GI Disorders – Advisory Committee/Board Member. Mahana Therapeutics, Owlstone Medical, Salix Pharmaceuticals, Vibrant Pharma – Consultant. Takeda Pharmaceuticals – Advisor or Review Panel Member, Consultant, Speaker. Vibrant Gastro – Advisor or Review Panel Member, Consultant, Speaker.
Darren M.. Brenner, MD. P5110 - Sustained Efficacy and Tolerability of Vibrant Capsule Therapy in a 77-Year-Old Female With Chronic Constipation: A 24-Month Case Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
