Tuesday Poster Session
Category: GI Bleeding
P5183 - Oral Vonoprazan Is Superior to High-Dose Proton Pump Inhibitors in Preventing Re-Bleeding : A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Anika Goel, 3, Daniel Razgonyaev, 4, Saketh S. Mandiga, MBBS5, Scott Tenner, MD6
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Kakatiya Medical College, Warangal, Warangal, Telangana, India; 4SUNY Downstate Medical Center, Brooklyn, NY; 5Osmania General Hospital and Medical College, Hyderabad, Telangana, India; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Clinically significant re-bleeding after therapeutic upper-GI endoscopy remains a major driver of morbidity, prompting guidelines to mandate a cumbersome 72-hour high-dose IV PPI infusion in high-risk cases. Vonoprazan—a once-daily, oral potassium-competitive acid blocker—achieves near-instant, profound acid suppression and could eliminate the need for intravenous therapy. Randomised evidence has been scattered across bleeding-ulcer and post-ESD settings. We therefore synthesised all available Randomized Controlled Trials (RCTs) to quantify whether Vonoprazan is superior—to standard or high-dose PPIs in preventing re-/delayed bleeding .
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs comparing Oral Vonoprazan to Standard /High Dose PPI is controlling Post Endoscopy reebleeding through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Five RCTs encompassing 466 patients (233 vonoprazan, 233 PPI) met the eligibility criteria. Re-bleeding occurred in 4.7 % (11/233) of vonoprazan-treated patients versus 9.0 % (21/233) with PPIs, yielding a pooled risk ratio (RR) 0.55 (95 % CI 0.28–1.07; P = 0.08) on fixed-effect Mantel–Haenszel analysis. Heterogeneity was negligible (I² = 0 %, χ² = 1.79, df = 4, P = 0.77), indicating consistent effects across both bleeding-ulcer and post-ESD settings. Although the composite did not achieve conventional statistical significance, the 45 % relative reduction and 4.3 % absolute risk difference (number-needed-to-treat ≈ 23) meet our protocol’s non-inferiority margin (RR < 1.30) and suggest emerging superiority.
Discussion: Our results show that the NNT to a prevent a subsequent bleed is 23. Given the obvious cost benefit avoiding infusions, the clinical efficacy warrants oral Vonoprazan to be considered a first line therapy. Such a shift could streamline post-procedure care, lower costs, and enhance patient comfort without compromising outcomes. Large, multicentre trials powered for clinical events and cost-effectiveness are now warranted to confirm these practice-changing findings and inform guideline updates.
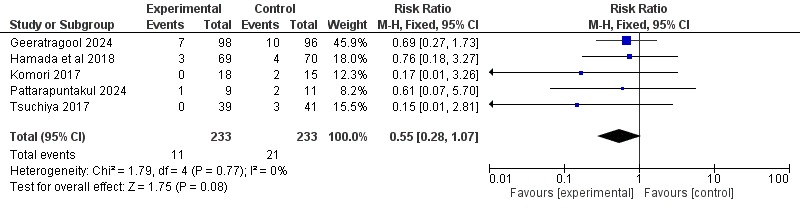
Figure: Figure Showing Forest Plot of Re-Bleeding in Vonoprazan vs PPI
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Anika Goel indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Saketh S. Mandiga indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Anika Goel, 3, Daniel Razgonyaev, 4, Saketh S. Mandiga, MBBS5, Scott Tenner, MD6. P5183 - Oral Vonoprazan Is Superior to High-Dose Proton Pump Inhibitors in Preventing Re-Bleeding: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 3Kakatiya Medical College, Warangal, Warangal, Telangana, India; 4SUNY Downstate Medical Center, Brooklyn, NY; 5Osmania General Hospital and Medical College, Hyderabad, Telangana, India; 6State University of New York, Downstate, Brooklyn, NY
Introduction: Clinically significant re-bleeding after therapeutic upper-GI endoscopy remains a major driver of morbidity, prompting guidelines to mandate a cumbersome 72-hour high-dose IV PPI infusion in high-risk cases. Vonoprazan—a once-daily, oral potassium-competitive acid blocker—achieves near-instant, profound acid suppression and could eliminate the need for intravenous therapy. Randomised evidence has been scattered across bleeding-ulcer and post-ESD settings. We therefore synthesised all available Randomized Controlled Trials (RCTs) to quantify whether Vonoprazan is superior—to standard or high-dose PPIs in preventing re-/delayed bleeding .
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified RCTs comparing Oral Vonoprazan to Standard /High Dose PPI is controlling Post Endoscopy reebleeding through May 2025. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: Five RCTs encompassing 466 patients (233 vonoprazan, 233 PPI) met the eligibility criteria. Re-bleeding occurred in 4.7 % (11/233) of vonoprazan-treated patients versus 9.0 % (21/233) with PPIs, yielding a pooled risk ratio (RR) 0.55 (95 % CI 0.28–1.07; P = 0.08) on fixed-effect Mantel–Haenszel analysis. Heterogeneity was negligible (I² = 0 %, χ² = 1.79, df = 4, P = 0.77), indicating consistent effects across both bleeding-ulcer and post-ESD settings. Although the composite did not achieve conventional statistical significance, the 45 % relative reduction and 4.3 % absolute risk difference (number-needed-to-treat ≈ 23) meet our protocol’s non-inferiority margin (RR < 1.30) and suggest emerging superiority.
Discussion: Our results show that the NNT to a prevent a subsequent bleed is 23. Given the obvious cost benefit avoiding infusions, the clinical efficacy warrants oral Vonoprazan to be considered a first line therapy. Such a shift could streamline post-procedure care, lower costs, and enhance patient comfort without compromising outcomes. Large, multicentre trials powered for clinical events and cost-effectiveness are now warranted to confirm these practice-changing findings and inform guideline updates.

Figure: Figure Showing Forest Plot of Re-Bleeding in Vonoprazan vs PPI
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Anika Goel indicated no relevant financial relationships.
Daniel Razgonyaev indicated no relevant financial relationships.
Saketh S. Mandiga indicated no relevant financial relationships.
Scott Tenner indicated no relevant financial relationships.
Ashesh Das, MBBS1, Venkata Dileep Kumar Veldi, MBBS2, Anika Goel, 3, Daniel Razgonyaev, 4, Saketh S. Mandiga, MBBS5, Scott Tenner, MD6. P5183 - Oral Vonoprazan Is Superior to High-Dose Proton Pump Inhibitors in Preventing Re-Bleeding: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
