Tuesday Poster Session
Category: IBD
P5347 - Single Arm Feasibility Trial of a Virtual Reality-Directed Brain-Gut Behavioral Pain Treatment for Hospitalized Patients With Inflammatory Bowel Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- BG
Brian Gutermuth, MD, MSc
University of Michigan
Ann Arbor, MI
Presenting Author(s)
Shirley Cohen-Mekelburg, MD, MS1, Brian Gutermuth, MD, MSc1, Gal Hodish, MPH1, John Sturgeon, PhD1, Ariel Jordan, MD1, Jessica Sheehan, MD1, Jeffrey Berinstein, MD, MS1, Melissa Dejonckheere, PhD1, Omer Liran, MD, MSHS2, Peter D. R.. Higgins, MD, PhD, MSc1
1University of Michigan, Ann Arbor, MI; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Hospitalized patients with inflammatory bowel disease (IBD) often receive inadequate pain treatment, given concerns for serious adverse effects from both opioid and non-opioid analgesics. Brain-gut behavioral therapies (BGBT) such as cognitive-behavioral therapy are effective for pain management but have not been fully examined as a pain treatment for hospitalized IBD patients. We examined the feasibility and acceptability of virtual reality (VR)-directed BGBT for hospitalized patients with IBD.
Methods: We conducted a prospective single-arm study of a multicomponent inpatient VR-BGBT (comprised of education, distraction games, breathing exercises, meditation, and cognitive-behavioral therapy) for patients with IBD. We recruited adult hospitalized patients with IBD who self-reported IBD-related pain (severity≥2, on a 0-10 scale). Our primary objective was intervention feasibility (the proportion of enrolled participants that received the VR-BGBT) and our secondary objective was intervention acceptability (evaluated using the Treatment Acceptability/Adherence Scale [TAAS] and qualitative interviews). We also explored changes in pain severity, perceived stress, pain catastrophizing, emotions, and IBD-related symptoms from baseline to post-treatment.
Results: We enrolled 23 participants who were hospitalized with IBD and self-reported pain (Table 1). We delivered the VR-BGBT to all 23 (100%) participants and 1 (4.4%) participant withdrew within 24 hours due to nausea, suggesting high tolerability of VR-BGBT. Participants rated the inpatient VR-BGBT treatment as acceptable (median TAAS 57, interquartile range [IQR] 52,62] on a 10-70 scale with higher scores indicating greater acceptability). Qualitative interviews revealed that VR-BGBT was easy to use, reduced the stress of hospitalization, distracted from acute pain, and promoted use of breathing exercises and meditation. Pre-to-post-treatment assessments demonstrated significant reductions in pain severity, perceived stress, daily pain catastrophizing, and bowel symptoms (Table 2).
Discussion: VR-BGBT is feasible and acceptable as an inpatient treatment option for patients with IBD-related pain. Preliminary study findings, while confounded by concomitant effective treatment for inflammation, suggest that VR-BGBT may address pain-related distress and will inform future efficacy studies.
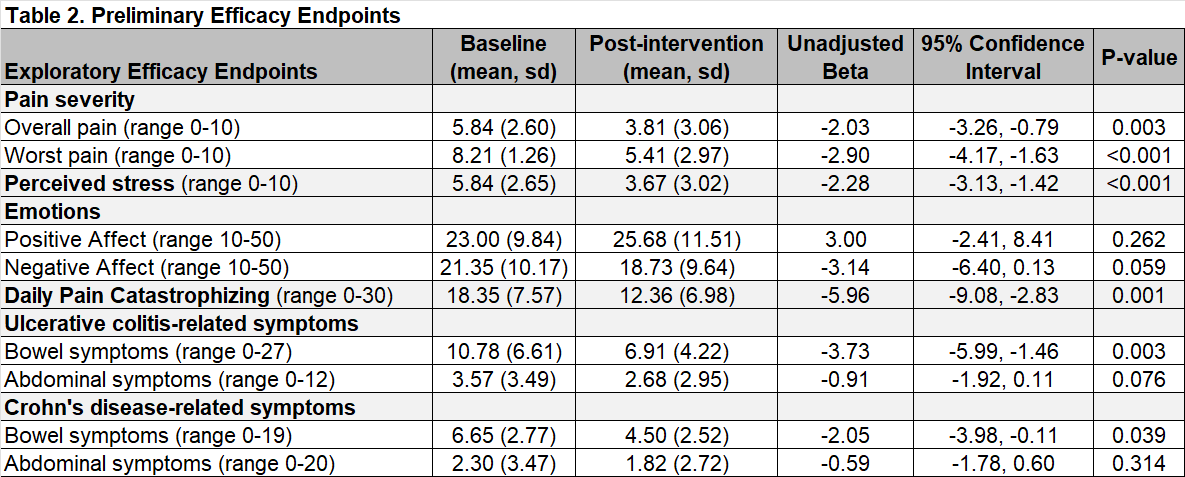
Figure: Table 1. Participant Characteristics
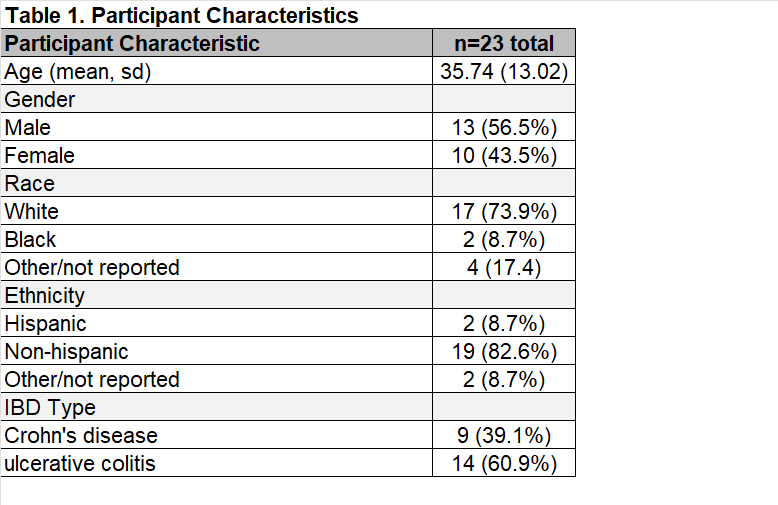
Figure: Table 2. Preliminary Efficacy Endpoints
Disclosures:
Shirley Cohen-Mekelburg indicated no relevant financial relationships.
Brian Gutermuth indicated no relevant financial relationships.
Gal Hodish indicated no relevant financial relationships.
John Sturgeon indicated no relevant financial relationships.
Ariel Jordan indicated no relevant financial relationships.
Jessica Sheehan indicated no relevant financial relationships.
Jeffrey Berinstein indicated no relevant financial relationships.
Melissa Dejonckheere indicated no relevant financial relationships.
Omer Liran: Xaia – Advisory Committee/Board Member, Royalties, Stock-privately held company.
Peter Higgins: AbbVie – Consultant.
Shirley Cohen-Mekelburg, MD, MS1, Brian Gutermuth, MD, MSc1, Gal Hodish, MPH1, John Sturgeon, PhD1, Ariel Jordan, MD1, Jessica Sheehan, MD1, Jeffrey Berinstein, MD, MS1, Melissa Dejonckheere, PhD1, Omer Liran, MD, MSHS2, Peter D. R.. Higgins, MD, PhD, MSc1. P5347 - Single Arm Feasibility Trial of a Virtual Reality-Directed Brain-Gut Behavioral Pain Treatment for Hospitalized Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Michigan, Ann Arbor, MI; 2Cedars-Sinai Medical Center, Los Angeles, CA
Introduction: Hospitalized patients with inflammatory bowel disease (IBD) often receive inadequate pain treatment, given concerns for serious adverse effects from both opioid and non-opioid analgesics. Brain-gut behavioral therapies (BGBT) such as cognitive-behavioral therapy are effective for pain management but have not been fully examined as a pain treatment for hospitalized IBD patients. We examined the feasibility and acceptability of virtual reality (VR)-directed BGBT for hospitalized patients with IBD.
Methods: We conducted a prospective single-arm study of a multicomponent inpatient VR-BGBT (comprised of education, distraction games, breathing exercises, meditation, and cognitive-behavioral therapy) for patients with IBD. We recruited adult hospitalized patients with IBD who self-reported IBD-related pain (severity≥2, on a 0-10 scale). Our primary objective was intervention feasibility (the proportion of enrolled participants that received the VR-BGBT) and our secondary objective was intervention acceptability (evaluated using the Treatment Acceptability/Adherence Scale [TAAS] and qualitative interviews). We also explored changes in pain severity, perceived stress, pain catastrophizing, emotions, and IBD-related symptoms from baseline to post-treatment.
Results: We enrolled 23 participants who were hospitalized with IBD and self-reported pain (Table 1). We delivered the VR-BGBT to all 23 (100%) participants and 1 (4.4%) participant withdrew within 24 hours due to nausea, suggesting high tolerability of VR-BGBT. Participants rated the inpatient VR-BGBT treatment as acceptable (median TAAS 57, interquartile range [IQR] 52,62] on a 10-70 scale with higher scores indicating greater acceptability). Qualitative interviews revealed that VR-BGBT was easy to use, reduced the stress of hospitalization, distracted from acute pain, and promoted use of breathing exercises and meditation. Pre-to-post-treatment assessments demonstrated significant reductions in pain severity, perceived stress, daily pain catastrophizing, and bowel symptoms (Table 2).
Discussion: VR-BGBT is feasible and acceptable as an inpatient treatment option for patients with IBD-related pain. Preliminary study findings, while confounded by concomitant effective treatment for inflammation, suggest that VR-BGBT may address pain-related distress and will inform future efficacy studies.

Figure: Table 1. Participant Characteristics

Figure: Table 2. Preliminary Efficacy Endpoints
Disclosures:
Shirley Cohen-Mekelburg indicated no relevant financial relationships.
Brian Gutermuth indicated no relevant financial relationships.
Gal Hodish indicated no relevant financial relationships.
John Sturgeon indicated no relevant financial relationships.
Ariel Jordan indicated no relevant financial relationships.
Jessica Sheehan indicated no relevant financial relationships.
Jeffrey Berinstein indicated no relevant financial relationships.
Melissa Dejonckheere indicated no relevant financial relationships.
Omer Liran: Xaia – Advisory Committee/Board Member, Royalties, Stock-privately held company.
Peter Higgins: AbbVie – Consultant.
Shirley Cohen-Mekelburg, MD, MS1, Brian Gutermuth, MD, MSc1, Gal Hodish, MPH1, John Sturgeon, PhD1, Ariel Jordan, MD1, Jessica Sheehan, MD1, Jeffrey Berinstein, MD, MS1, Melissa Dejonckheere, PhD1, Omer Liran, MD, MSHS2, Peter D. R.. Higgins, MD, PhD, MSc1. P5347 - Single Arm Feasibility Trial of a Virtual Reality-Directed Brain-Gut Behavioral Pain Treatment for Hospitalized Patients With Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
