Tuesday Poster Session
Category: IBD
P5344 - Characterizing Clinical Outcomes Between Accelerated vs Standard Dose Infliximab in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Anil Bhatnagar, MD
Penn State Health Milton S. Hershey Medical Center
Hershey, PA
Presenting Author(s)
Anil Bhatnagar, MD1, Shivani Mattikalli, MD2, Paddy Ssentongo, MD, PhD1, Justin Canakis, DO1, Andrew Tinsley, MD, MS3, Emmanuelle D. Williams, MD, MSEd4, Kofi Clarke, MD1, Matthew Coates, MD, PhD5
1Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 2University of Maryland Medical Center, Baltimore, MD; 3Penn State Health, Hummelstown, PA; 4Penn State Health Milton S. Hershey Medical Center, Elizabethtown, PA; 5Penn State College of Medicine, Hershey, PA
Introduction: Infliximab may be used to treat moderate-to-severe ulcerative colitis (UC). It may also induce remission in patients with acute severe ulcerative colitis. In some cases, a clinician may choose to treat with accelerated induction, with shorter intervals for loading doses and/or with higher doses. This meta-analysis evaluated efficacy and safety of accelerated vs. standard dose infliximab, and expands on existing literature by evaluating additional clinical endpoints, including clinical remission, adverse effects, infection rate, and length of hospital stay.
Methods: Medline, OVID, and Cochrane Library were searched from inception to 11/30/2024 for randomized clinical trials (RCTs) and retrospective chart reviews reporting the comparison of standard vs. accelerated infliximab induction therapy for patients with acute severe ulcerative colitis. Standard dosing was defined as 5mg/kg with infusions at week 0, 2, and 6 while accelerated dosing was defined as either intensified dosing (greater than 5mg/kg) or as a shortened induction timeline. We estimated the effect of these two treatment strategies by random-effects meta-analyses using the generic inverse variance method. Results were reported as prevalence (%) of outcomes in each treatment group. Lengths of hospital stay was reported in days.
Results: Eighteen eligible studies evaluating a total of 2371 patients with severe UC were included (1336 in the standard cohort, and 1022 in the accelerated cohort). Median age was 37 years (interquartile range (IQR): 29-41), and 60% (IQR: 52% to 62%) were male. No significant difference was found in the clinical remission rate when comparing the accelerated (47%; 95% CI: 32% to 63%) and standard dose infliximab cohorts (53%; 95% CI: 39% to 67%) (Figure 1a, Figure 1b). Additionally, there were no significant differences in infection rates (22%; 95% CI: 10% to 42% vs. 31%; 95% CI: 23% to 41%), adverse events (18%; 95% CI: 13% to 24% vs. 16%; 95% CI: 9% to 28%) or length of hospital stay (14 days vs. 13 days) between the cohorts. The risk of bias assessment was moderate.
Discussion: In this systematic review and meta-analysis of RCTs and retrospective chart reviews, patients receiving accelerated dose infliximab did not demonstrate significant differences in efficacy-related outcomes when compared to those receiving standard dosing. However, there were also no significant differences in safety endpoints between these cohorts.
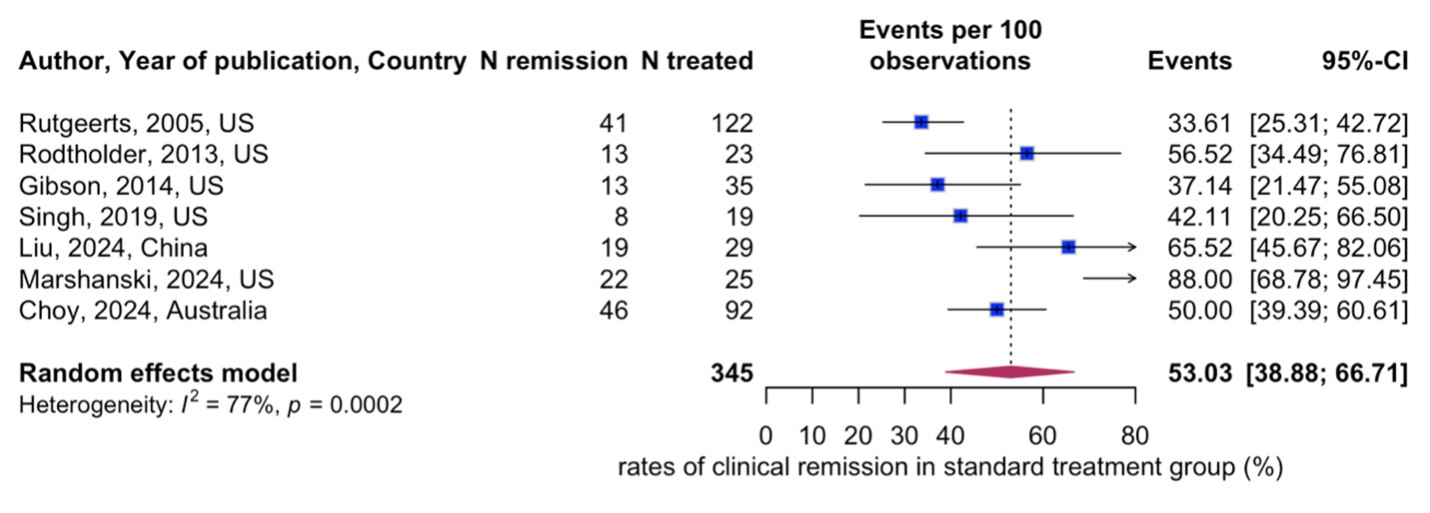
Figure: Figure 1a. Accelerated treatment group – Remission
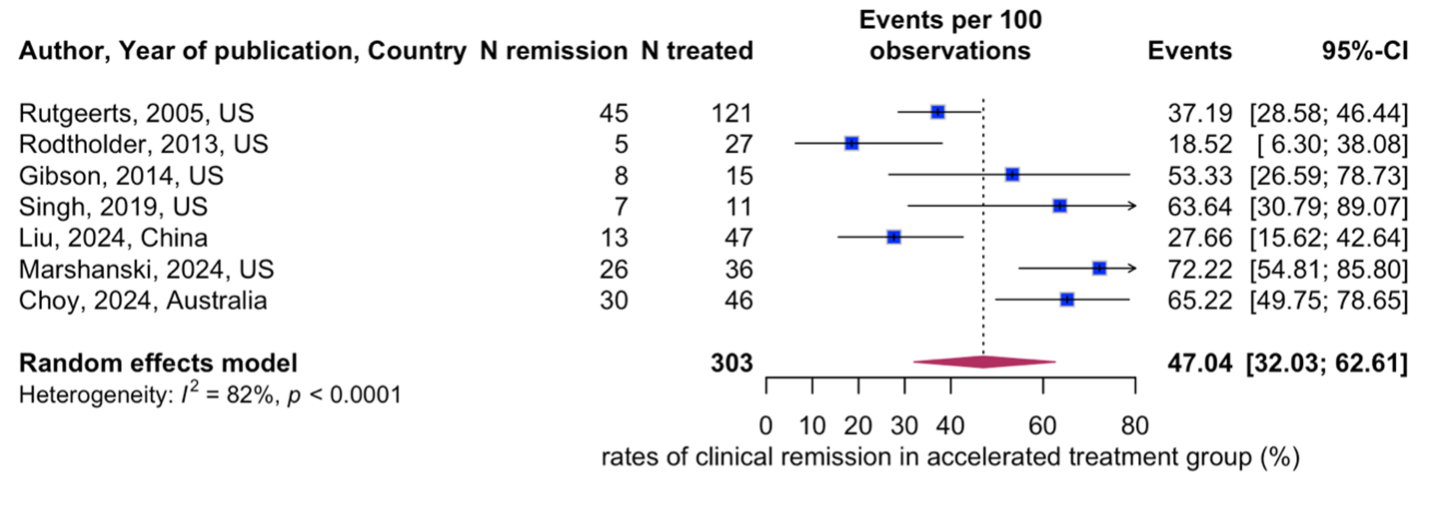
Figure: Figure 1b. Standard treatment group – Remission
Disclosures:
Anil Bhatnagar indicated no relevant financial relationships.
Shivani Mattikalli indicated no relevant financial relationships.
Paddy Ssentongo indicated no relevant financial relationships.
Justin Canakis indicated no relevant financial relationships.
Andrew Tinsley: Abbvie – Speakers Bureau.
Emmanuelle Williams: Abbvie – Speakers Bureau. Pfizer – Speakers Bureau.
Kofi Clarke: Takeda – Clinical Trial Support.
Matthew Coates indicated no relevant financial relationships.
Anil Bhatnagar, MD1, Shivani Mattikalli, MD2, Paddy Ssentongo, MD, PhD1, Justin Canakis, DO1, Andrew Tinsley, MD, MS3, Emmanuelle D. Williams, MD, MSEd4, Kofi Clarke, MD1, Matthew Coates, MD, PhD5. P5344 - Characterizing Clinical Outcomes Between Accelerated vs Standard Dose Infliximab in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Penn State Health Milton S. Hershey Medical Center, Hershey, PA; 2University of Maryland Medical Center, Baltimore, MD; 3Penn State Health, Hummelstown, PA; 4Penn State Health Milton S. Hershey Medical Center, Elizabethtown, PA; 5Penn State College of Medicine, Hershey, PA
Introduction: Infliximab may be used to treat moderate-to-severe ulcerative colitis (UC). It may also induce remission in patients with acute severe ulcerative colitis. In some cases, a clinician may choose to treat with accelerated induction, with shorter intervals for loading doses and/or with higher doses. This meta-analysis evaluated efficacy and safety of accelerated vs. standard dose infliximab, and expands on existing literature by evaluating additional clinical endpoints, including clinical remission, adverse effects, infection rate, and length of hospital stay.
Methods: Medline, OVID, and Cochrane Library were searched from inception to 11/30/2024 for randomized clinical trials (RCTs) and retrospective chart reviews reporting the comparison of standard vs. accelerated infliximab induction therapy for patients with acute severe ulcerative colitis. Standard dosing was defined as 5mg/kg with infusions at week 0, 2, and 6 while accelerated dosing was defined as either intensified dosing (greater than 5mg/kg) or as a shortened induction timeline. We estimated the effect of these two treatment strategies by random-effects meta-analyses using the generic inverse variance method. Results were reported as prevalence (%) of outcomes in each treatment group. Lengths of hospital stay was reported in days.
Results: Eighteen eligible studies evaluating a total of 2371 patients with severe UC were included (1336 in the standard cohort, and 1022 in the accelerated cohort). Median age was 37 years (interquartile range (IQR): 29-41), and 60% (IQR: 52% to 62%) were male. No significant difference was found in the clinical remission rate when comparing the accelerated (47%; 95% CI: 32% to 63%) and standard dose infliximab cohorts (53%; 95% CI: 39% to 67%) (Figure 1a, Figure 1b). Additionally, there were no significant differences in infection rates (22%; 95% CI: 10% to 42% vs. 31%; 95% CI: 23% to 41%), adverse events (18%; 95% CI: 13% to 24% vs. 16%; 95% CI: 9% to 28%) or length of hospital stay (14 days vs. 13 days) between the cohorts. The risk of bias assessment was moderate.
Discussion: In this systematic review and meta-analysis of RCTs and retrospective chart reviews, patients receiving accelerated dose infliximab did not demonstrate significant differences in efficacy-related outcomes when compared to those receiving standard dosing. However, there were also no significant differences in safety endpoints between these cohorts.

Figure: Figure 1a. Accelerated treatment group – Remission

Figure: Figure 1b. Standard treatment group – Remission
Disclosures:
Anil Bhatnagar indicated no relevant financial relationships.
Shivani Mattikalli indicated no relevant financial relationships.
Paddy Ssentongo indicated no relevant financial relationships.
Justin Canakis indicated no relevant financial relationships.
Andrew Tinsley: Abbvie – Speakers Bureau.
Emmanuelle Williams: Abbvie – Speakers Bureau. Pfizer – Speakers Bureau.
Kofi Clarke: Takeda – Clinical Trial Support.
Matthew Coates indicated no relevant financial relationships.
Anil Bhatnagar, MD1, Shivani Mattikalli, MD2, Paddy Ssentongo, MD, PhD1, Justin Canakis, DO1, Andrew Tinsley, MD, MS3, Emmanuelle D. Williams, MD, MSEd4, Kofi Clarke, MD1, Matthew Coates, MD, PhD5. P5344 - Characterizing Clinical Outcomes Between Accelerated vs Standard Dose Infliximab in Ulcerative Colitis Patients: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
