Tuesday Poster Session
Category: IBD
P5338 - New Insights Into Pregnancy Outcomes and Inflammatory Bowel Disease: A Closer Look at Ustekinumab and Vedolizumab During Pregnancy
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- JA
Juhi Amin, MBBS, MS (she/her/hers)
Department of Obstetrics and Gynecology, Government Medical College and SSG Hospital, Baroda
Nadiad, Gujarat, India
Presenting Author(s)
Sanjiboni Das, MBBS1, Juhi Amin, MBBS, MS2, Sweta Sahu, MBBS3, Heom Mahendra Bhatt, MBBS4, Khyati Menghani, MBBS5, Shreya Kattela, MBBS6, Namra Gohil, MBBS7, Sravan K. Nekkanti, MD, BS8, Aayushi J. Rajani, MD9
1Dr. Mk.shah medical college and research centre, Gandhinagar, Gujarat, India; 2Department of Obstetrics and Gynecology, Government Medical College and SSG Hospital, Baroda, Vadodara, Gujarat, India; 3J.J.M. Medical College, Bhubaneswar, Orissa, India; 4Dr.M.K.Shah medical College and Research centre, Bagasara, Gujarat, India; 5University of Virginia, Charlottesville, VA; 6Bhaskar Medical College, Hyderabad, Telangana, India; 7Medical College Baroda, Vadodara, Gujarat, Vadodara, Gujarat, India; 8University of Louisville, Louisville, KY; 9University of Missouri Health Care, Columbia, MO
Introduction: Inflammatory Bowel Disease (IBD) presents unique challenges in pregnancy, particularly regarding management options. While the data remains limited, emerging evidence suggests that biologics such as Vedolizumab (VM) and Ustekinumab (UM) may be safe during pregnancy. In this review, we summarize current literature on maternal and neonatal outcomes associated with these therapies.
Methods: We conducted a review of literature using PubMed, PMC and related databases. Studies reporting pregnancy outcomes in IBD patients exposed to UM or VM were included. Data on key maternal and fetal outcomes was extracted. A meta-analysis was conducted to assess the risk of preterm birth with VM use. Additionally, we developed a conceptual decision tree to explore factors influencing elective abortion in patients with IBD taking VM/UM.
Results: Ten studies comprising 12,138 pregnancies met inclusion criteria. Across multiple prospective cohort studies and registry analyses, including data from the PIANO registry and multicenter European cohorts, both biologics were not associated with a significant increase in adverse pregnancy outcomes. UM exposure was associated with live birth rates of 80-89% and no clear increase in adverse outcomes was found. Similar findings were observed with VM. Although one meta-analysis of observational data reported a potential increase in preterm birth with VM (OR 2.16), this finding did not achieve statistical significance in the pooled analysis (summary OR 1.30; 95% CI: 0.88–1.92). Elective abortion rates ranged from 3–6%, with no clear evidence linking these decisions to drug exposure. To explore this further, we proposed a decision tree distinguishing drug-related from non-drug related motivations (Figure 1).
Discussion: Our findings support the relative safety of Ustekinumab and Vedolizumab during pregnancy, with no significant increase in congenital anomalies, spontaneous abortion, or NICU admissions. However, the reasons behind elective abortions in this population are likely multifactorial and remain understudied. We believe the proposed framework may help clinicians and researchers better understand the mix of medical, psychological, and informational factors influencing these choices. Importantly, all studies were observational; no randomized controlled trials (RCTs) are currently available. High-quality prospective research and ethically feasible RCTs are critically needed to provide definitive safety data for the use of these biologics in pregnancy.
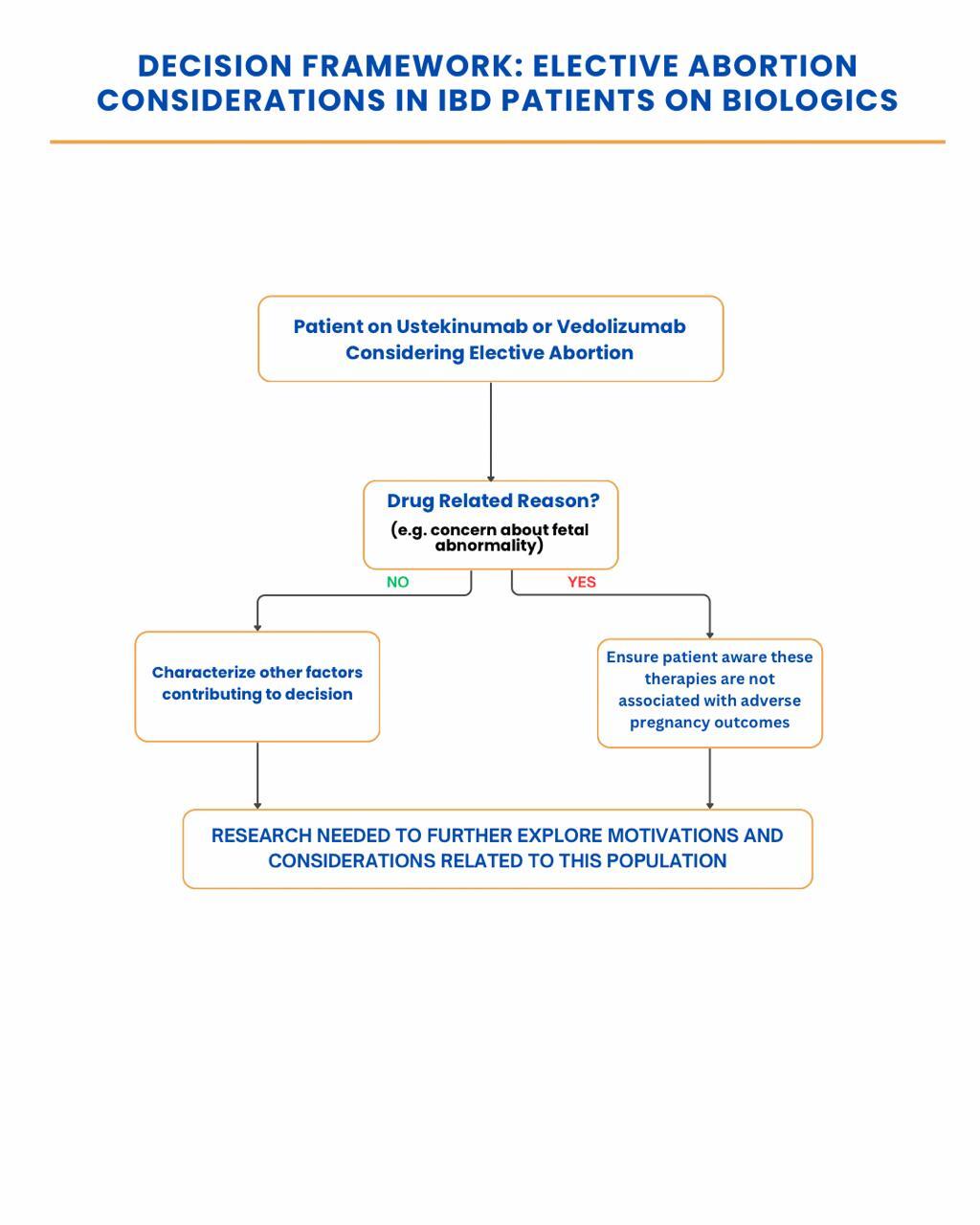
Figure: Figure 1
Disclosures:
Sanjiboni Das indicated no relevant financial relationships.
Juhi Amin indicated no relevant financial relationships.
Sweta Sahu indicated no relevant financial relationships.
Heom Mahendra Bhatt indicated no relevant financial relationships.
Khyati Menghani indicated no relevant financial relationships.
Shreya Kattela indicated no relevant financial relationships.
Namra Gohil indicated no relevant financial relationships.
Sravan Nekkanti indicated no relevant financial relationships.
Aayushi Rajani indicated no relevant financial relationships.
Sanjiboni Das, MBBS1, Juhi Amin, MBBS, MS2, Sweta Sahu, MBBS3, Heom Mahendra Bhatt, MBBS4, Khyati Menghani, MBBS5, Shreya Kattela, MBBS6, Namra Gohil, MBBS7, Sravan K. Nekkanti, MD, BS8, Aayushi J. Rajani, MD9. P5338 - New Insights Into Pregnancy Outcomes and Inflammatory Bowel Disease: A Closer Look at Ustekinumab and Vedolizumab During Pregnancy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Dr. Mk.shah medical college and research centre, Gandhinagar, Gujarat, India; 2Department of Obstetrics and Gynecology, Government Medical College and SSG Hospital, Baroda, Vadodara, Gujarat, India; 3J.J.M. Medical College, Bhubaneswar, Orissa, India; 4Dr.M.K.Shah medical College and Research centre, Bagasara, Gujarat, India; 5University of Virginia, Charlottesville, VA; 6Bhaskar Medical College, Hyderabad, Telangana, India; 7Medical College Baroda, Vadodara, Gujarat, Vadodara, Gujarat, India; 8University of Louisville, Louisville, KY; 9University of Missouri Health Care, Columbia, MO
Introduction: Inflammatory Bowel Disease (IBD) presents unique challenges in pregnancy, particularly regarding management options. While the data remains limited, emerging evidence suggests that biologics such as Vedolizumab (VM) and Ustekinumab (UM) may be safe during pregnancy. In this review, we summarize current literature on maternal and neonatal outcomes associated with these therapies.
Methods: We conducted a review of literature using PubMed, PMC and related databases. Studies reporting pregnancy outcomes in IBD patients exposed to UM or VM were included. Data on key maternal and fetal outcomes was extracted. A meta-analysis was conducted to assess the risk of preterm birth with VM use. Additionally, we developed a conceptual decision tree to explore factors influencing elective abortion in patients with IBD taking VM/UM.
Results: Ten studies comprising 12,138 pregnancies met inclusion criteria. Across multiple prospective cohort studies and registry analyses, including data from the PIANO registry and multicenter European cohorts, both biologics were not associated with a significant increase in adverse pregnancy outcomes. UM exposure was associated with live birth rates of 80-89% and no clear increase in adverse outcomes was found. Similar findings were observed with VM. Although one meta-analysis of observational data reported a potential increase in preterm birth with VM (OR 2.16), this finding did not achieve statistical significance in the pooled analysis (summary OR 1.30; 95% CI: 0.88–1.92). Elective abortion rates ranged from 3–6%, with no clear evidence linking these decisions to drug exposure. To explore this further, we proposed a decision tree distinguishing drug-related from non-drug related motivations (Figure 1).
Discussion: Our findings support the relative safety of Ustekinumab and Vedolizumab during pregnancy, with no significant increase in congenital anomalies, spontaneous abortion, or NICU admissions. However, the reasons behind elective abortions in this population are likely multifactorial and remain understudied. We believe the proposed framework may help clinicians and researchers better understand the mix of medical, psychological, and informational factors influencing these choices. Importantly, all studies were observational; no randomized controlled trials (RCTs) are currently available. High-quality prospective research and ethically feasible RCTs are critically needed to provide definitive safety data for the use of these biologics in pregnancy.

Figure: Figure 1
Disclosures:
Sanjiboni Das indicated no relevant financial relationships.
Juhi Amin indicated no relevant financial relationships.
Sweta Sahu indicated no relevant financial relationships.
Heom Mahendra Bhatt indicated no relevant financial relationships.
Khyati Menghani indicated no relevant financial relationships.
Shreya Kattela indicated no relevant financial relationships.
Namra Gohil indicated no relevant financial relationships.
Sravan Nekkanti indicated no relevant financial relationships.
Aayushi Rajani indicated no relevant financial relationships.
Sanjiboni Das, MBBS1, Juhi Amin, MBBS, MS2, Sweta Sahu, MBBS3, Heom Mahendra Bhatt, MBBS4, Khyati Menghani, MBBS5, Shreya Kattela, MBBS6, Namra Gohil, MBBS7, Sravan K. Nekkanti, MD, BS8, Aayushi J. Rajani, MD9. P5338 - New Insights Into Pregnancy Outcomes and Inflammatory Bowel Disease: A Closer Look at Ustekinumab and Vedolizumab During Pregnancy, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
