Tuesday Poster Session
Category: IBD
P5337 - Safety and Weight Loss Outcomes of Tirzepatide in Obesity Patients With Inflammatory Bowel Disease: A Matched-Control Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Jose Villamarin, MD
Mayo Clinic
Rochester, MN
Presenting Author(s)
Jose Villamarin, MD1, Maria Antonia Espinosa, MD1, Thomas Fredrick, MD1, Regina Castaneda, MD2, Rene J. Rivera Gutierrez, MD2, Dima Bechenati, MD1, Diego Anazco, MD1, Wissam Ghusn, MD1, Maria D. Hurtado, MD2, Andres Acosta, MD1
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Jacksonville, FL
Introduction: Tirzepatide, a dual GLP-1 and GIP agonist, is a promising anti-obesity medication. While clinical trials demonstrate significant weight loss efficacy, it is still unknown if it is safe and effective in inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis. Here, we aimed to assess the safety and efficacy of tirzepatide in patients with IBD.
Methods: This retrospective cohort study evaluated patients with obesity and inflammatory bowel disease (IBD) receiving tirzepatide for weight management compared to a matched control group without IBD. A 2:1 propensity score matching was utilized, resulting in 29 patients with IBD and 58 controls without IBD. Matching criteria included demographic and baseline characteristics: age, gender, race, baseline weight, and body mass index (BMI). Primary outcomes were side effects and total body weight loss (TBWL%) at 3, 6, 9, 12, and 15 months. Statistical analyses used independent t-tests for continuous variables and Fisher's exact tests for categorical variables.
Results: The cohort included 87 patients (mean age 58 ± 7 years; BMI 36 ± 3 kg/m²; 60% female; 85% white). After propensity score adjustment, no significant differences between groups. Weight loss outcomes (TBWL%) were similar between groups at all time points. Mean TBWL% at 12 months was -10.4 ± 8.4% (IBD) vs -8.7 ± 7.4% (control, p=0.42), and at last follow-up (15 months) was -12.1 ± 8.8% (IBD) vs -12.0 ± 8.9% (control, p=0.96). Overall side effects were numerically higher in the IBD group (58%) compared to controls (27%, p=0.009). IBD patients experienced higher rates of nausea/vomiting (41 % vs. 10 %; p = 0.003), abdominal pain (20 % vs. 3 %; p = 0.01), and fatigue (10 % vs. 0%; p = 0.03).
Discussion: We show that obesity patients with IBD treated with tirzepatide experience similar weight loss outcomes but significantly more side effects , particularly gastrointestinal symptoms, compared to matched controls without IBD. Larger prospective studies are needed to better understand the long-term safety and efficacy of tirzepatide in this population.
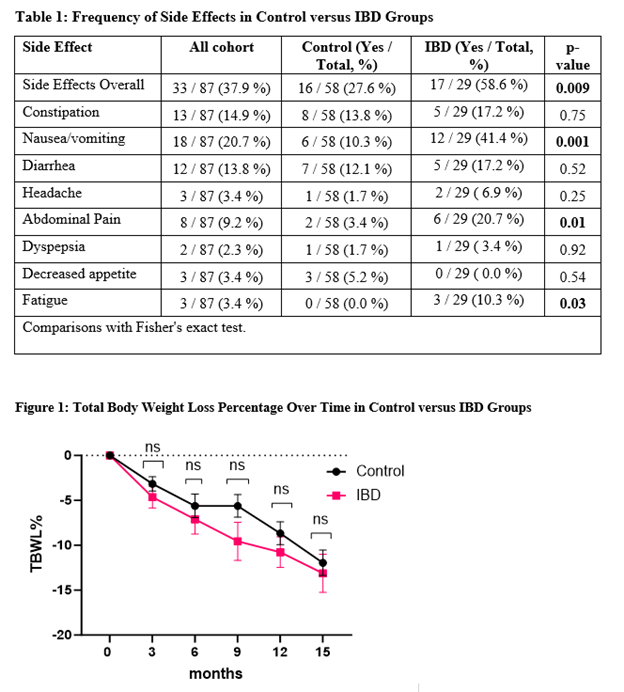
Figure: Table 1: Frequency of Side Effects in Control versus IBD Groups. Figure 1: Total Body Weight Loss Percentage Over Time in Control versus IBD Groups
Disclosures:
Jose Villamarin indicated no relevant financial relationships.
Maria Antonia Espinosa indicated no relevant financial relationships.
Thomas Fredrick indicated no relevant financial relationships.
Regina Castaneda indicated no relevant financial relationships.
Rene Rivera Gutierrez indicated no relevant financial relationships.
Dima Bechenati indicated no relevant financial relationships.
Diego Anazco indicated no relevant financial relationships.
Wissam Ghusn indicated no relevant financial relationships.
Maria D. Hurtado: NovoNordisk – Advisor or Review Panel Member, funding from the National Institute of Health (K12-AR084222), the Mayo Clinic Center for Women's Health Research, and Phenomix Sciences.
Andres Acosta: Phenomix sciences – Royalties. Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences – Consultant, Grant/Research Support.
Jose Villamarin, MD1, Maria Antonia Espinosa, MD1, Thomas Fredrick, MD1, Regina Castaneda, MD2, Rene J. Rivera Gutierrez, MD2, Dima Bechenati, MD1, Diego Anazco, MD1, Wissam Ghusn, MD1, Maria D. Hurtado, MD2, Andres Acosta, MD1. P5337 - Safety and Weight Loss Outcomes of Tirzepatide in Obesity Patients With Inflammatory Bowel Disease: A Matched-Control Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Rochester, MN; 2Mayo Clinic, Jacksonville, FL
Introduction: Tirzepatide, a dual GLP-1 and GIP agonist, is a promising anti-obesity medication. While clinical trials demonstrate significant weight loss efficacy, it is still unknown if it is safe and effective in inflammatory bowel disease (IBD), such as Crohn’s disease and ulcerative colitis. Here, we aimed to assess the safety and efficacy of tirzepatide in patients with IBD.
Methods: This retrospective cohort study evaluated patients with obesity and inflammatory bowel disease (IBD) receiving tirzepatide for weight management compared to a matched control group without IBD. A 2:1 propensity score matching was utilized, resulting in 29 patients with IBD and 58 controls without IBD. Matching criteria included demographic and baseline characteristics: age, gender, race, baseline weight, and body mass index (BMI). Primary outcomes were side effects and total body weight loss (TBWL%) at 3, 6, 9, 12, and 15 months. Statistical analyses used independent t-tests for continuous variables and Fisher's exact tests for categorical variables.
Results: The cohort included 87 patients (mean age 58 ± 7 years; BMI 36 ± 3 kg/m²; 60% female; 85% white). After propensity score adjustment, no significant differences between groups. Weight loss outcomes (TBWL%) were similar between groups at all time points. Mean TBWL% at 12 months was -10.4 ± 8.4% (IBD) vs -8.7 ± 7.4% (control, p=0.42), and at last follow-up (15 months) was -12.1 ± 8.8% (IBD) vs -12.0 ± 8.9% (control, p=0.96). Overall side effects were numerically higher in the IBD group (58%) compared to controls (27%, p=0.009). IBD patients experienced higher rates of nausea/vomiting (41 % vs. 10 %; p = 0.003), abdominal pain (20 % vs. 3 %; p = 0.01), and fatigue (10 % vs. 0%; p = 0.03).
Discussion: We show that obesity patients with IBD treated with tirzepatide experience similar weight loss outcomes but significantly more side effects , particularly gastrointestinal symptoms, compared to matched controls without IBD. Larger prospective studies are needed to better understand the long-term safety and efficacy of tirzepatide in this population.

Figure: Table 1: Frequency of Side Effects in Control versus IBD Groups. Figure 1: Total Body Weight Loss Percentage Over Time in Control versus IBD Groups
Disclosures:
Jose Villamarin indicated no relevant financial relationships.
Maria Antonia Espinosa indicated no relevant financial relationships.
Thomas Fredrick indicated no relevant financial relationships.
Regina Castaneda indicated no relevant financial relationships.
Rene Rivera Gutierrez indicated no relevant financial relationships.
Dima Bechenati indicated no relevant financial relationships.
Diego Anazco indicated no relevant financial relationships.
Wissam Ghusn indicated no relevant financial relationships.
Maria D. Hurtado: NovoNordisk – Advisor or Review Panel Member, funding from the National Institute of Health (K12-AR084222), the Mayo Clinic Center for Women's Health Research, and Phenomix Sciences.
Andres Acosta: Phenomix sciences – Royalties. Rhythm Pharmaceuticals, Gila Therapeutics, Amgen, General Mills, Regeneron, Boehringer Ingelheim, Novo Nordisk, Currax, Nestlé, Phenomix Sciences – Consultant, Grant/Research Support.
Jose Villamarin, MD1, Maria Antonia Espinosa, MD1, Thomas Fredrick, MD1, Regina Castaneda, MD2, Rene J. Rivera Gutierrez, MD2, Dima Bechenati, MD1, Diego Anazco, MD1, Wissam Ghusn, MD1, Maria D. Hurtado, MD2, Andres Acosta, MD1. P5337 - Safety and Weight Loss Outcomes of Tirzepatide in Obesity Patients With Inflammatory Bowel Disease: A Matched-Control Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
