Tuesday Poster Session
Category: IBD
P5431 - Efficacy of Retreatment With Upadacitinib After Treatment Interruption in Ulcerative Colitis: Data From the U-ACTIVATE Open-Label Extension
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Remo Panaccione, MD (he/him/his)
Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada
Calgary, AB, Canada
Presenting Author(s)
Award: ACG Presidential Poster Award
Remo Panaccione, MD1, Jean-Frederic Colombel, MD2, Marla Dubinsky, MD2, Christopher Ma, MD, MPH3, Tadakazu Hisamatsu, MD, PhD4, Michelle Kujawski, PhD5, Erica Cheng, PhD5, Elena Dubcenco, MD, MS5, Sina Ogholikhan, MD5, Elena Marced Barrachina, MD, MBA5, Smitha Suravaram, MD5, Severine Vermeire, MD, PhD6
1Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3University of Calgary, Calgary, AB, Canada; 4Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 5AbbVie Inc., North Chicago, IL; 6Department of Gastroenterology & Hepatology, University hospital Leuven, Leuven, Brabant Wallon, Belgium
Introduction: Upadacitinib (UPA) is an oral, reversible Janus kinase inhibitor (JAKi) approved for the treatment of moderate-to-severe ulcerative colitis (UC). In clinical practice, patients may temporarily discontinue JAKis and then restart treatment for varying reasons; however, the efficacy of UPA in this scenario has not been fully characterized. Here, we assess the efficacy of UPA in patients with UC who lost response when UPA treatment was interrupted and then retreated with the lowest dose through 144 weeks (wks).
Methods: This analysis of the U-ACTIVATE (NCT04988386) open-label extension (OLE) included the subset of patients in the U-ACHIEVE or U-ACCOMPLISH studies who responded to 8 wks of UPA 45 mg once daily (QD) induction therapy and were then re-randomized to placebo (PBO) for maintenance. Patients who subsequently lost response on PBO entered the OLE and were treated with UPA 15 mg QD (UPA15); those who had inadequate response on UPA15 were then escalated to UPA30 (Figure 1). Clinical remission per adapted Mayo score and partial adapted Mayo score, endoscopic improvement, and endoscopic remission were assessed at wks 48, 96, and 144. Safety was not assessed in this analysis but has been reported previously (Panaccione et al. J Crohns Colitis. 2024;18[Suppl 1]:i1821–2).
Results: A total of 110 patients who lost response after UPA 45 mg induction and withdrawal to placebo entered the U-ACTIVATE OLE and were treated with UPA15; at wk144, 57 remained on UPA15 (UPA15àUPA15), 47 were escalated to UPA30 (UPA15àUPA30), and 6 were de-escalated to UPA15 after the escalation and thus were not included in this analysis. Clinical remission per adapted Mayo score was achieved by 52.4%, 66.7%, and 76.3% of patients at wks 48, 96, and 144, respectively, in the UPA15àUPA15 group and 48.3%, 59.4%, and 61.1% of patients in the UPA15àUPA30 group (Fig. 2A). Most patients in both UPA treatment groups achieved clinical remission per partial adapted Mayo score all time points (Fig. 2B). Similar results were observed for endoscopic improvement (Fig. 2C). Endoscopic remission was achieved by 31.3%, 40.0%, and 33.3% of patients at wks 48, 96, and 144, respectively for UPA15àUPA15 and 30.0%, 56.3%, and 56.4% of patients for UPA15àUPA30 (Fig. 2D).
Discussion: In most patients with UC who lost response following temporary UPA treatment interruption, clinical and endoscopic efficacy were recaptured following treatment with UPA15 and/or escalation to UPA30 in case of inadequate response in the U-ACTIVATE OLE.
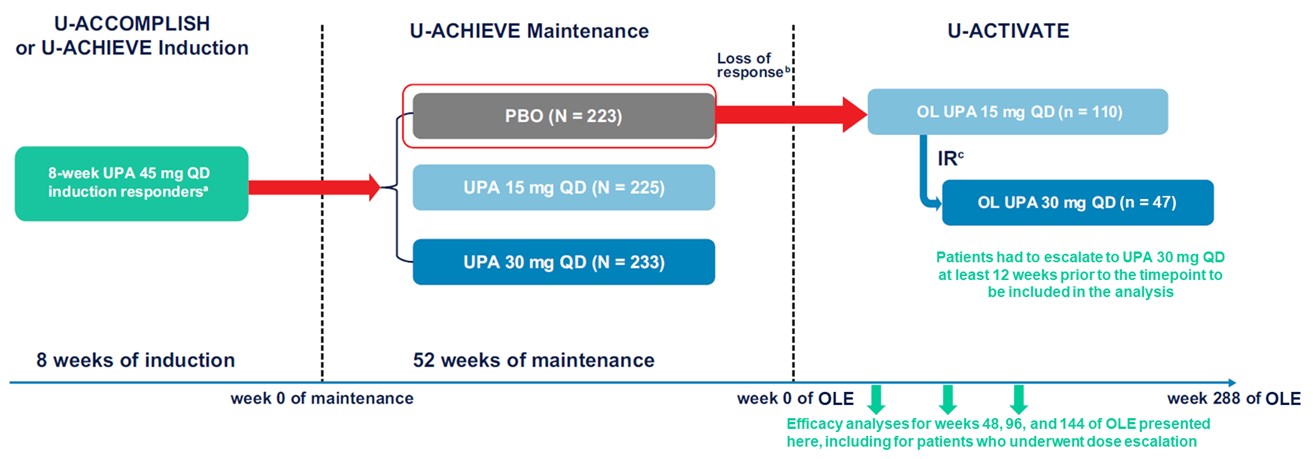
Figure: Figure 1. Study Design Schematic.
Abbreviations: IR, inadequate response; OL, open-label; OLE, open-label extension; PBO, placebo; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UPA, upadacitinib.
aClinical response was defined as a decrease in adapted Mayo score of ≥ 2 points and ≥ 30% from baseline, plus a decrease in RBS of ≥ 1 or an absolute RBS of ≤ 1.
bAmong patients with mean daily SFS and RBS < 2.1 at maintenance week 0: an SFS and RBS each ≥ 1-point greater than the maintenance week 0 value on 2 consecutive visits ≥ 14 days apart. Among patients with SFS or RBS ≥ 2.1 at maintenance week 0: either an SFS or RBS ≥ 1‑point greater than the maintenance week 0 value on 2 consecutive visits ≥ 14 days apart.
cPatients with an inadequate response (SFS + RBS that is unchanged or has increased from week 0 on 2 consecutive visits ≥ 7 days apart) to UPA 15 mg QD could be dose escalated to UPA 30 mg QD between weeks 2 and 36 of the OLE.
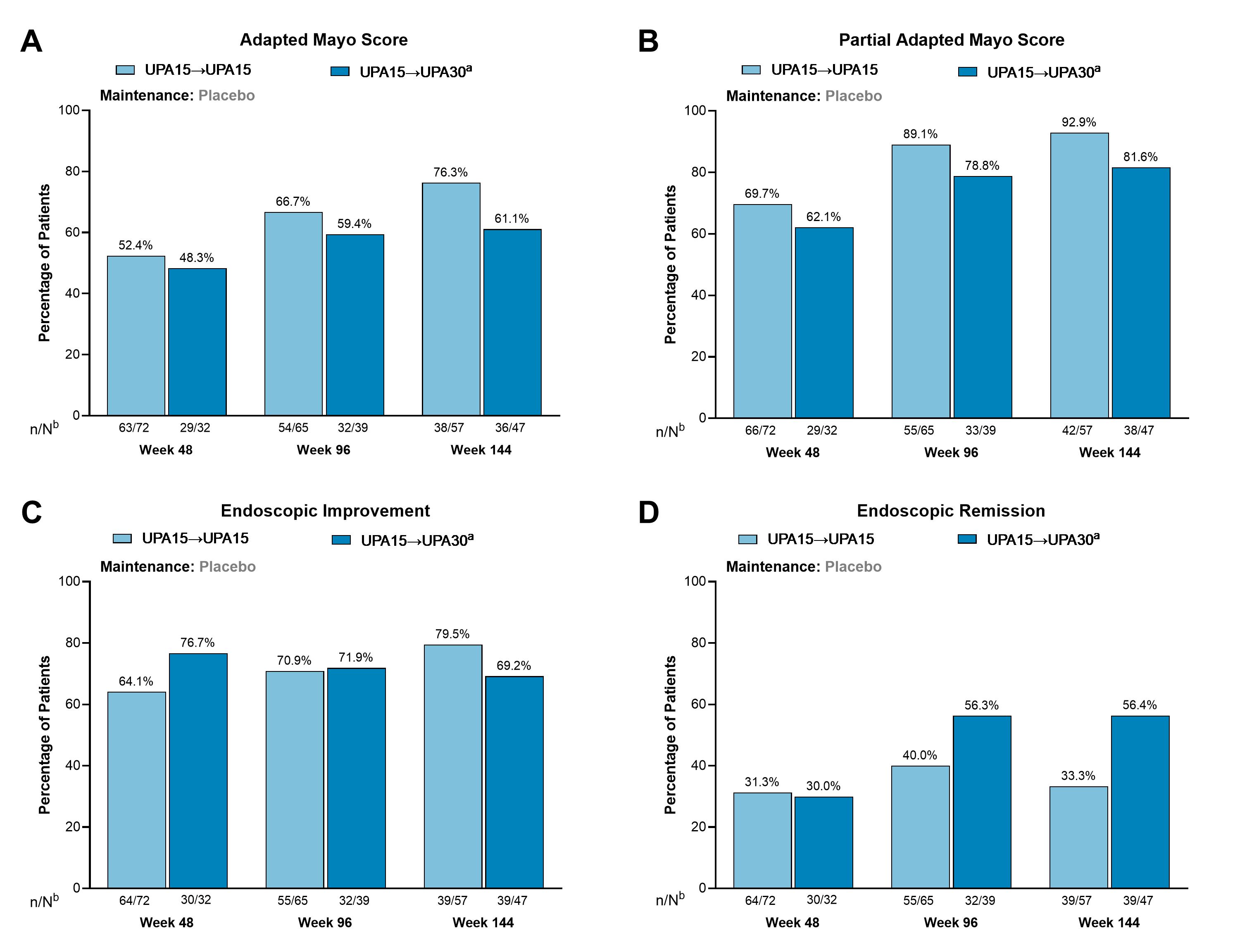
Figure: Figure 2. Among Patients With a Loss of Response After Temporary Treatment Interruption During Maintenance, Clinical Remission, Endoscopic Improvement, and Endoscopic Remission Were Recaptured After 48, 96, and 144 Weeks.
Abbreviations: n, number of patients with available data; N, total number of patients on study treatment; OLE, open-label extension; RBS, rectal bleeding score; SFS, stool frequency subscore; UPA, upadacitinib.
Clinical remission per adapted Mayo score is defined as an SFS ≤ 1 and not greater than baseline, RBS = 0, and endoscopy subscore ≤ 1 without friability. Clinical remission per partial adapted Mayo score is defined as an SFS ≤ 1 and RBS = 0. Endoscopic improvement is defined as an endoscopic subscore of ≤ 1. Endoscopic remission is defined as an endoscopic subscore of 0.
Patient population: Patients randomized to UPA 45 mg during induction who achieved response at week 8 and were then randomized to placebo during maintenance. Patients lost response during maintenance and entered the OLE on UPA15. Patients who flared twice within 4 weeks (2 weeks apart) were escalated to treatment with UPA30.
aPatients with an inadequate response to UPA15, defined as an SFS + RBS that was unchanged or increased from week 0 on 2 consecutive visits ≥ 7 days apart, were escalated to UPA30. Patients had to escalate at least 12 weeks prior to the timepoint.
bN values are patient counts based on escalation status through Week 36 for evaluation at Week 48, Week 84 for evaluation at Week 96, and Week 132 for evaluation at Week 144.
Disclosures:
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Jean-Frederic Colombel: AbbVie – Grant/Research Support, Personal fees. Amgen – Personal fees. Boehringer Ingelheim – Personal fees. Celgene – Personal fees. Celltrion – Personal fees. Enterome – Personal fees. Ferring – Personal fees. Genentech – Personal fees. Genfit – Stock Options. Intestinal Biotech Development – Stock Options. Janssen – Grant/Research Support, Personal fees. MedImmune – Consultant, Personal fees. Merck – Personal fees. Pfizer – Personal fees. PPM Services – Personal fees. Protagonist – Personal fees. Second Genome – Personal fees. Seres – Personal fees. Shire – Personal fees. Takeda – Grant/Research Support, Personal fees. Theradiag – Personal fees.
Marla Dubinsky: AbbVie – Advisor or Review Panel Member, Consultant. Abivax – Advisor or Review Panel Member, Consultant. AstraZeneca – Advisor or Review Panel Member, Consultant. Boehringer Ingelheim – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Celltrion – Advisor or Review Panel Member, Consultant. Eli Lilly – Advisor or Review Panel Member, Consultant. Genentech – Advisor or Review Panel Member, Consultant. Gilead – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Consultant. Merck – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Prometheus Biosciences – Advisor or Review Panel Member, Consultant. Prometheus Labs – Advisor or Review Panel Member, Consultant. Roche – Advisor or Review Panel Member, Consultant. Sanofi – Advisor or Review Panel Member, Consultant. Spyre Therapeutics – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Christopher Ma: Abbvie – Consultant, Grant/Research Support, speaker's fee. Alimentiv – Consultant, speaker's fee. Amgen – Consultant, speaker's fee. AVIR Pharma Inc. – Consultant, speaker's fee. Bristol Myers Squibb – Consultant, speaker's fee. Celltrion – Consultant. Eli Lilly – Consultant, Grant/Research Support, speaker's fee. Ferring – Consultant, Grant/Research Support, speaker's fee. Forte Biosciences – Consultant. Fresenius Kabi – Consultant, speaker's fee. Gilead – Consultant. Janssen – Consultant, speaker's fee. McKesson – Consultant. Merck – Speaker's fee. Mirador Therapeutics – Consultant. Mylan – Consultant. Organon – speaker's fee. Pendopharm – Consultant, speaker's fee. Pfizer – Consultant, Grant/Research Support, speaker's fee. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant, speaker's fee. Springer Publishing – Royalties. Takeda – Consultant, speaker's fee. Tillotts Pharma – Consultant, speaker's fee.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Michelle Kujawski: AbbVie – Employee, Stock Options.
Erica Cheng: AbbVie – Employee, Stock Options.
Elena Dubcenco: AbbVie – Employee, Stock Options.
Sina Ogholikhan: AbbVie – Employee, Stock Options.
Elena Marced Barrachina: AbbVie – Employee, Stock Options.
Smitha Suravaram: AbbVie – Employee, Stock Options.
Severine Vermeire: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AbolerIS Pharma – Consultant, Speakers Bureau. AgomAb – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Avaxia – Consultant, Speakers Bureau. BMS – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech-Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIdomics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. Mestag Therapeutics, – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prodigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillots Pharma AG – Consultant, Speakers Bureau. VectivBio – Consultant, Speakers Bureau. Ventyx – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Remo Panaccione, MD1, Jean-Frederic Colombel, MD2, Marla Dubinsky, MD2, Christopher Ma, MD, MPH3, Tadakazu Hisamatsu, MD, PhD4, Michelle Kujawski, PhD5, Erica Cheng, PhD5, Elena Dubcenco, MD, MS5, Sina Ogholikhan, MD5, Elena Marced Barrachina, MD, MBA5, Smitha Suravaram, MD5, Severine Vermeire, MD, PhD6. P5431 - Efficacy of Retreatment With Upadacitinib After Treatment Interruption in Ulcerative Colitis: Data From the U-ACTIVATE Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Remo Panaccione, MD1, Jean-Frederic Colombel, MD2, Marla Dubinsky, MD2, Christopher Ma, MD, MPH3, Tadakazu Hisamatsu, MD, PhD4, Michelle Kujawski, PhD5, Erica Cheng, PhD5, Elena Dubcenco, MD, MS5, Sina Ogholikhan, MD5, Elena Marced Barrachina, MD, MBA5, Smitha Suravaram, MD5, Severine Vermeire, MD, PhD6
1Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada; 2Icahn School of Medicine at Mount Sinai, New York, NY; 3University of Calgary, Calgary, AB, Canada; 4Department of Gastroenterology and Hepatology, Kyorin University School of Medicine, Tokyo, Tokyo, Japan; 5AbbVie Inc., North Chicago, IL; 6Department of Gastroenterology & Hepatology, University hospital Leuven, Leuven, Brabant Wallon, Belgium
Introduction: Upadacitinib (UPA) is an oral, reversible Janus kinase inhibitor (JAKi) approved for the treatment of moderate-to-severe ulcerative colitis (UC). In clinical practice, patients may temporarily discontinue JAKis and then restart treatment for varying reasons; however, the efficacy of UPA in this scenario has not been fully characterized. Here, we assess the efficacy of UPA in patients with UC who lost response when UPA treatment was interrupted and then retreated with the lowest dose through 144 weeks (wks).
Methods: This analysis of the U-ACTIVATE (NCT04988386) open-label extension (OLE) included the subset of patients in the U-ACHIEVE or U-ACCOMPLISH studies who responded to 8 wks of UPA 45 mg once daily (QD) induction therapy and were then re-randomized to placebo (PBO) for maintenance. Patients who subsequently lost response on PBO entered the OLE and were treated with UPA 15 mg QD (UPA15); those who had inadequate response on UPA15 were then escalated to UPA30 (Figure 1). Clinical remission per adapted Mayo score and partial adapted Mayo score, endoscopic improvement, and endoscopic remission were assessed at wks 48, 96, and 144. Safety was not assessed in this analysis but has been reported previously (Panaccione et al. J Crohns Colitis. 2024;18[Suppl 1]:i1821–2).
Results: A total of 110 patients who lost response after UPA 45 mg induction and withdrawal to placebo entered the U-ACTIVATE OLE and were treated with UPA15; at wk144, 57 remained on UPA15 (UPA15àUPA15), 47 were escalated to UPA30 (UPA15àUPA30), and 6 were de-escalated to UPA15 after the escalation and thus were not included in this analysis. Clinical remission per adapted Mayo score was achieved by 52.4%, 66.7%, and 76.3% of patients at wks 48, 96, and 144, respectively, in the UPA15àUPA15 group and 48.3%, 59.4%, and 61.1% of patients in the UPA15àUPA30 group (Fig. 2A). Most patients in both UPA treatment groups achieved clinical remission per partial adapted Mayo score all time points (Fig. 2B). Similar results were observed for endoscopic improvement (Fig. 2C). Endoscopic remission was achieved by 31.3%, 40.0%, and 33.3% of patients at wks 48, 96, and 144, respectively for UPA15àUPA15 and 30.0%, 56.3%, and 56.4% of patients for UPA15àUPA30 (Fig. 2D).
Discussion: In most patients with UC who lost response following temporary UPA treatment interruption, clinical and endoscopic efficacy were recaptured following treatment with UPA15 and/or escalation to UPA30 in case of inadequate response in the U-ACTIVATE OLE.

Figure: Figure 1. Study Design Schematic.
Abbreviations: IR, inadequate response; OL, open-label; OLE, open-label extension; PBO, placebo; QD, once daily; RBS, rectal bleeding subscore; SFS, stool frequency subscore; UPA, upadacitinib.
aClinical response was defined as a decrease in adapted Mayo score of ≥ 2 points and ≥ 30% from baseline, plus a decrease in RBS of ≥ 1 or an absolute RBS of ≤ 1.
bAmong patients with mean daily SFS and RBS < 2.1 at maintenance week 0: an SFS and RBS each ≥ 1-point greater than the maintenance week 0 value on 2 consecutive visits ≥ 14 days apart. Among patients with SFS or RBS ≥ 2.1 at maintenance week 0: either an SFS or RBS ≥ 1‑point greater than the maintenance week 0 value on 2 consecutive visits ≥ 14 days apart.
cPatients with an inadequate response (SFS + RBS that is unchanged or has increased from week 0 on 2 consecutive visits ≥ 7 days apart) to UPA 15 mg QD could be dose escalated to UPA 30 mg QD between weeks 2 and 36 of the OLE.

Figure: Figure 2. Among Patients With a Loss of Response After Temporary Treatment Interruption During Maintenance, Clinical Remission, Endoscopic Improvement, and Endoscopic Remission Were Recaptured After 48, 96, and 144 Weeks.
Abbreviations: n, number of patients with available data; N, total number of patients on study treatment; OLE, open-label extension; RBS, rectal bleeding score; SFS, stool frequency subscore; UPA, upadacitinib.
Clinical remission per adapted Mayo score is defined as an SFS ≤ 1 and not greater than baseline, RBS = 0, and endoscopy subscore ≤ 1 without friability. Clinical remission per partial adapted Mayo score is defined as an SFS ≤ 1 and RBS = 0. Endoscopic improvement is defined as an endoscopic subscore of ≤ 1. Endoscopic remission is defined as an endoscopic subscore of 0.
Patient population: Patients randomized to UPA 45 mg during induction who achieved response at week 8 and were then randomized to placebo during maintenance. Patients lost response during maintenance and entered the OLE on UPA15. Patients who flared twice within 4 weeks (2 weeks apart) were escalated to treatment with UPA30.
aPatients with an inadequate response to UPA15, defined as an SFS + RBS that was unchanged or increased from week 0 on 2 consecutive visits ≥ 7 days apart, were escalated to UPA30. Patients had to escalate at least 12 weeks prior to the timepoint.
bN values are patient counts based on escalation status through Week 36 for evaluation at Week 48, Week 84 for evaluation at Week 96, and Week 132 for evaluation at Week 144.
Disclosures:
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Jean-Frederic Colombel: AbbVie – Grant/Research Support, Personal fees. Amgen – Personal fees. Boehringer Ingelheim – Personal fees. Celgene – Personal fees. Celltrion – Personal fees. Enterome – Personal fees. Ferring – Personal fees. Genentech – Personal fees. Genfit – Stock Options. Intestinal Biotech Development – Stock Options. Janssen – Grant/Research Support, Personal fees. MedImmune – Consultant, Personal fees. Merck – Personal fees. Pfizer – Personal fees. PPM Services – Personal fees. Protagonist – Personal fees. Second Genome – Personal fees. Seres – Personal fees. Shire – Personal fees. Takeda – Grant/Research Support, Personal fees. Theradiag – Personal fees.
Marla Dubinsky: AbbVie – Advisor or Review Panel Member, Consultant. Abivax – Advisor or Review Panel Member, Consultant. AstraZeneca – Advisor or Review Panel Member, Consultant. Boehringer Ingelheim – Advisor or Review Panel Member, Consultant. Bristol Myers Squibb – Advisor or Review Panel Member, Consultant. Celltrion – Advisor or Review Panel Member, Consultant. Eli Lilly – Advisor or Review Panel Member, Consultant. Genentech – Advisor or Review Panel Member, Consultant. Gilead – Advisor or Review Panel Member, Consultant. Janssen – Advisor or Review Panel Member, Consultant. Merck – Advisor or Review Panel Member, Consultant. Pfizer – Advisor or Review Panel Member, Consultant. Prometheus Biosciences – Advisor or Review Panel Member, Consultant. Prometheus Labs – Advisor or Review Panel Member, Consultant. Roche – Advisor or Review Panel Member, Consultant. Sanofi – Advisor or Review Panel Member, Consultant. Spyre Therapeutics – Advisor or Review Panel Member, Consultant. Takeda – Advisor or Review Panel Member, Consultant.
Christopher Ma: Abbvie – Consultant, Grant/Research Support, speaker's fee. Alimentiv – Consultant, speaker's fee. Amgen – Consultant, speaker's fee. AVIR Pharma Inc. – Consultant, speaker's fee. Bristol Myers Squibb – Consultant, speaker's fee. Celltrion – Consultant. Eli Lilly – Consultant, Grant/Research Support, speaker's fee. Ferring – Consultant, Grant/Research Support, speaker's fee. Forte Biosciences – Consultant. Fresenius Kabi – Consultant, speaker's fee. Gilead – Consultant. Janssen – Consultant, speaker's fee. McKesson – Consultant. Merck – Speaker's fee. Mirador Therapeutics – Consultant. Mylan – Consultant. Organon – speaker's fee. Pendopharm – Consultant, speaker's fee. Pfizer – Consultant, Grant/Research Support, speaker's fee. Prometheus Biosciences Inc. – Consultant. Roche – Consultant. Sanofi – Consultant, speaker's fee. Springer Publishing – Royalties. Takeda – Consultant, speaker's fee. Tillotts Pharma – Consultant, speaker's fee.
Tadakazu Hisamatsu: AbbVie GK – Consultant, Grant/Research Support, Lecture fees. Boston Scientific Corporation – Grant/Research Support. Bristol Myers Squibb – Consultant. Daiichi Sankyo Co. Ltd. – Grant/Research Support, Honararium. EA Pharma Co. Ltd. – Consultant, Grant/Research Support, Lecture fees. Gilead Sciences – Consultant. JIMRO Co. Ltd. – Grant/Research Support, Lecture fees. Johnson & Johnson – Consultant, Lecture fees. Kissei Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Kyorin Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Lilly – Consultant. Mitsubishi Tanabe Pharma Corporation – Consultant, Grant/Research Support, Lecture fees. Mochida Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Nichi-Iko Pharmaceutical Co. Ltd – Consultant. Nippon Kayaku Co. Ltd. – Grant/Research Support. Pfizer Inc. – Consultant, Grant/Research Support, Lecture fees. Takeda Pharmaceutical Co. Ltd. – Grant/Research Support, Lecture fees. Zeria Pharmaceutical Co – Grant/Research Support.
Michelle Kujawski: AbbVie – Employee, Stock Options.
Erica Cheng: AbbVie – Employee, Stock Options.
Elena Dubcenco: AbbVie – Employee, Stock Options.
Sina Ogholikhan: AbbVie – Employee, Stock Options.
Elena Marced Barrachina: AbbVie – Employee, Stock Options.
Smitha Suravaram: AbbVie – Employee, Stock Options.
Severine Vermeire: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Abivax – Consultant, Speakers Bureau. AbolerIS Pharma – Consultant, Speakers Bureau. AgomAb – Consultant, Speakers Bureau. Alimentiv – Consultant, Speakers Bureau. Arena Pharmaceuticals – Consultant, Speakers Bureau. AstraZeneca – Consultant, Speakers Bureau. Avaxia – Consultant, Speakers Bureau. BMS – Consultant, Speakers Bureau. Boehringer Ingelheim – Consultant, Speakers Bureau. Celgene – Consultant, Speakers Bureau. CVasThera – Consultant, Speakers Bureau. Cytoki Pharma – Consultant, Speakers Bureau. Dr Falk Pharma – Consultant, Speakers Bureau. Eli Lilly and Company – Consultant, Speakers Bureau. Ferring – Consultant, Speakers Bureau. Galapagos – Consultant, Grant/Research Support, Speakers Bureau. Genentech-Roche – Consultant, Speakers Bureau. Gilead – Consultant, Speakers Bureau. GSK – Consultant, Speakers Bureau. Hospira – Consultant, Speakers Bureau. IMIdomics – Consultant, Speakers Bureau. Janssen – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Grant/Research Support, Speakers Bureau. Materia Prima – Consultant, Speakers Bureau. Mestag Therapeutics, – Consultant, Speakers Bureau. MiroBio – Consultant, Speakers Bureau. Morphic – Consultant, Speakers Bureau. MRM Health – Consultant, Speakers Bureau. MSD – Consultant, Speakers Bureau. Mundipharma – Consultant, Speakers Bureau. Pfizer – Consultant, Grant/Research Support, Speakers Bureau. Prodigest – Consultant, Speakers Bureau. Progenity – Consultant, Speakers Bureau. Prometheus – Consultant, Speakers Bureau. Robarts Clinical Trials – Consultant, Speakers Bureau. Second Genome – Consultant, Speakers Bureau. Shire – Consultant, Speakers Bureau. Surrozen – Consultant, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Theravance Biopharma – Consultant, Speakers Bureau. Tillots Pharma AG – Consultant, Speakers Bureau. VectivBio – Consultant, Speakers Bureau. Ventyx – Consultant, Speakers Bureau. Zealand Pharma – Consultant, Speakers Bureau.
Remo Panaccione, MD1, Jean-Frederic Colombel, MD2, Marla Dubinsky, MD2, Christopher Ma, MD, MPH3, Tadakazu Hisamatsu, MD, PhD4, Michelle Kujawski, PhD5, Erica Cheng, PhD5, Elena Dubcenco, MD, MS5, Sina Ogholikhan, MD5, Elena Marced Barrachina, MD, MBA5, Smitha Suravaram, MD5, Severine Vermeire, MD, PhD6. P5431 - Efficacy of Retreatment With Upadacitinib After Treatment Interruption in Ulcerative Colitis: Data From the U-ACTIVATE Open-Label Extension, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

