Tuesday Poster Session
Category: IBD
P5429 - Efficacy and Safety of Infliximab for Management of Immune-Related Adverse Events (irAEs): A Retrospective Multi-irAE Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- FK
Filippos Koutroumpakis, MD
The University of Texas MD Anderson Cancer Center
Houston, TX
Presenting Author(s)
Filippos Koutroumpakis, MD1, Kevin Shi, MD2, Kei Takigawa, MD3, Irene Lee, MD3, Anirudha Chatterjee, MD4, Sidra Naz, MD, MPH5, Yinghong Wang, MD, PhD, MS5
1The University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas at Houston, Houston, TX; 3Baylor College of Medicine, Houston, TX; 4University of Texas Medical Branch, Galveston, TX; 5University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Immune-related adverse events (irAEs) are frequent complications of immune checkpoint inhibitor (ICI) therapy and often require immunosuppressive treatment. Infliximab is commonly used for steroid-refractory irAEs, yet real-world data across irAE subtypes remain limited. We investigated the efficacy and safety of infliximab across diverse irAEs.
Methods: We retrospectively analyzed patients treated with ICIs at a tertiary cancer center between 2013 and 2024 who developed irAEs requiring infliximab. Patients were categorized based on the infliximab cycles and doses. The Common Terminology Criteria for Adverse Events developed by the American Society of Clinical Oncology was used to evaluate severity and define treatment outcomes. Symptom improvement to grade 1 or complete resolution was considered clinical remission; improvement by one grade was classified as partial response. Adverse events related to infliximab were recorded. Chi-square and Wilcoxon tests were used for categorical and continuous variables, respectively.
Results: Among 450 patients included (median age 65, 36% female), common malignancies included melanoma (49%), genitourinary (24%), and pulmonary (12%). The most frequent irAEs were gastrointestinal (59%), pulmonary (19%), and renal (12%). The median number of infliximab 5 mg/kg doses was 1 (IQR 1–2) (Table 1). Among patients receiving 5 mg/kg, clinical remission or response was achieved in 63% of those given 1–2 doses versus 95% of those receiving ≥3 doses. No significant differences were observed between the 1–2 and ≥3 dose groups in peak CTCAE toxicity grade, steroid duration, biologic switching, recurrence or new irAEs, or infliximab-related adverse events (Table 2). Five patients began infliximab directly at 10 mg/kg, and two escalated from 5 to 10 mg/kg due to lack of response. Six of seven patients received 1–2 doses. Three achieved clinical remission, two achieved clinical response, and two had treatment failure.
Discussion: Infliximab at the dose of 5 mg/kg as the most used dosage, is effective and well-tolerated for diverse steroid-refractory irAEs. A higher dose of 10mg/kg is rarely needed and does not show greater therapeutic benefit. A regimen of ≥3 doses is associated with higher remission rates without increased adverse events, supporting its use as a standard therapeutic approach across irAE types. Further studies are needed to compare infliximab with other first-line immunosuppressive treatments.
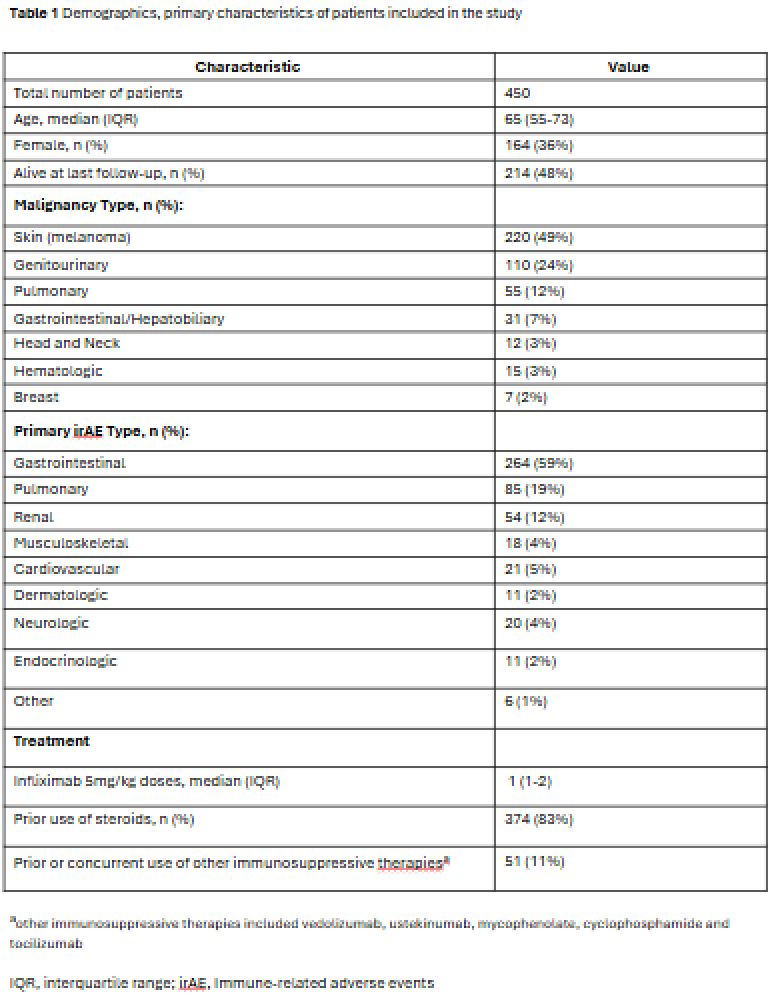
Figure: Table 1
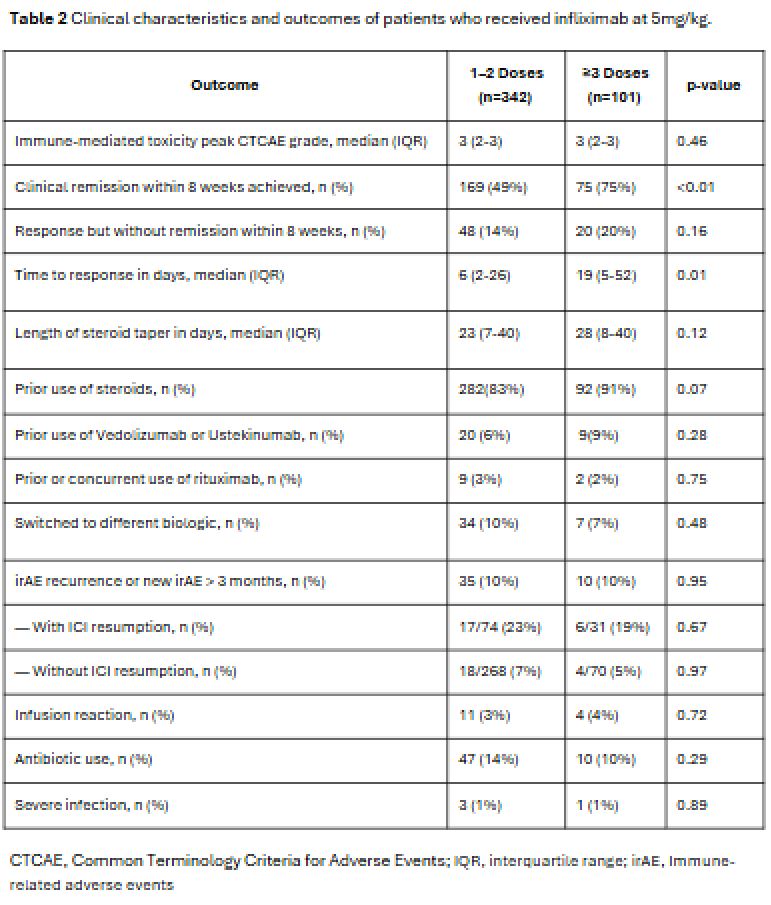
Figure: Table 2
Disclosures:
Filippos Koutroumpakis indicated no relevant financial relationships.
Kevin Shi indicated no relevant financial relationships.
Kei Takigawa indicated no relevant financial relationships.
Irene Lee indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Filippos Koutroumpakis, MD1, Kevin Shi, MD2, Kei Takigawa, MD3, Irene Lee, MD3, Anirudha Chatterjee, MD4, Sidra Naz, MD, MPH5, Yinghong Wang, MD, PhD, MS5. P5429 - Efficacy and Safety of Infliximab for Management of Immune-Related Adverse Events (irAEs): A Retrospective Multi-irAE Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1The University of Texas MD Anderson Cancer Center, Houston, TX; 2University of Texas at Houston, Houston, TX; 3Baylor College of Medicine, Houston, TX; 4University of Texas Medical Branch, Galveston, TX; 5University of Texas MD Anderson Cancer Center, Houston, TX
Introduction: Immune-related adverse events (irAEs) are frequent complications of immune checkpoint inhibitor (ICI) therapy and often require immunosuppressive treatment. Infliximab is commonly used for steroid-refractory irAEs, yet real-world data across irAE subtypes remain limited. We investigated the efficacy and safety of infliximab across diverse irAEs.
Methods: We retrospectively analyzed patients treated with ICIs at a tertiary cancer center between 2013 and 2024 who developed irAEs requiring infliximab. Patients were categorized based on the infliximab cycles and doses. The Common Terminology Criteria for Adverse Events developed by the American Society of Clinical Oncology was used to evaluate severity and define treatment outcomes. Symptom improvement to grade 1 or complete resolution was considered clinical remission; improvement by one grade was classified as partial response. Adverse events related to infliximab were recorded. Chi-square and Wilcoxon tests were used for categorical and continuous variables, respectively.
Results: Among 450 patients included (median age 65, 36% female), common malignancies included melanoma (49%), genitourinary (24%), and pulmonary (12%). The most frequent irAEs were gastrointestinal (59%), pulmonary (19%), and renal (12%). The median number of infliximab 5 mg/kg doses was 1 (IQR 1–2) (Table 1). Among patients receiving 5 mg/kg, clinical remission or response was achieved in 63% of those given 1–2 doses versus 95% of those receiving ≥3 doses. No significant differences were observed between the 1–2 and ≥3 dose groups in peak CTCAE toxicity grade, steroid duration, biologic switching, recurrence or new irAEs, or infliximab-related adverse events (Table 2). Five patients began infliximab directly at 10 mg/kg, and two escalated from 5 to 10 mg/kg due to lack of response. Six of seven patients received 1–2 doses. Three achieved clinical remission, two achieved clinical response, and two had treatment failure.
Discussion: Infliximab at the dose of 5 mg/kg as the most used dosage, is effective and well-tolerated for diverse steroid-refractory irAEs. A higher dose of 10mg/kg is rarely needed and does not show greater therapeutic benefit. A regimen of ≥3 doses is associated with higher remission rates without increased adverse events, supporting its use as a standard therapeutic approach across irAE types. Further studies are needed to compare infliximab with other first-line immunosuppressive treatments.

Figure: Table 1

Figure: Table 2
Disclosures:
Filippos Koutroumpakis indicated no relevant financial relationships.
Kevin Shi indicated no relevant financial relationships.
Kei Takigawa indicated no relevant financial relationships.
Irene Lee indicated no relevant financial relationships.
Anirudha Chatterjee indicated no relevant financial relationships.
Sidra Naz indicated no relevant financial relationships.
Yinghong Wang indicated no relevant financial relationships.
Filippos Koutroumpakis, MD1, Kevin Shi, MD2, Kei Takigawa, MD3, Irene Lee, MD3, Anirudha Chatterjee, MD4, Sidra Naz, MD, MPH5, Yinghong Wang, MD, PhD, MS5. P5429 - Efficacy and Safety of Infliximab for Management of Immune-Related Adverse Events (irAEs): A Retrospective Multi-irAE Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
