Tuesday Poster Session
Category: IBD
P5428 - Trends in Out-of-Pocket Spending for Drugs Under Medicare Part D in Moderate to Severe Inflammatory Bowel Disease and the Impact of the Inflation Reduction Act
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Mythili Menon Pathiyil, MD
Saint Vincent Hospital
Worcester, MA
Presenting Author(s)
Mythili Menon Pathiyil, MD1, Arvind Kumar Venkataramana Raju, MD1, Parth Patel, MD2, Rutwik Pradeep. Sharma, MD3, Victor Chedid, MD, MSc4
1Saint Vincent Hospital, Worcester, MA; 2Ascension Saint Joseph Hospital, Chicago, IL; 3University of Michigan-Sparrow Hospital, Lansing, MI; 4Mayo Clinic, Rochester, MN
Introduction: Treatments for moderate to severe inflammatory bowel disease (IBD) include biologics or small molecules. Most are branded with limited generic substitution, resulting in high out-of-pocket (OOP) expenses that can lead to premature drug discontinuation. We aimed to assess trends in OOP costs for key IBD therapies covered under Medicare Part D and evaluate the financial impact of the Inflation Reduction Act (IRA) $2,000 OOP cap implemented in 2025.
Methods: We analyzed Medicare Part D Prescription Drug Plan Formulary and Pricing Files from Q3 (July–September) of 2019–2024 and Q1 of 2025. Eight available IBD drugs were included: adalimumab, tofacitinib, upadacitinib, ustekinumab, risankizumab, guselkumab, certolizumab and golimumab. Annual retail and OOP costs were estimated (maintenance doses for ustekinumab and risankizumab and both induction and maintenance doses for the rest) per drug using Medicare Part D benefit structures for each year. Costs were inflation-adjusted to 2025 U.S. dollars using a standardized conversion factor. Data analysis was performed in Stata 18; time trends were visualized using R.
Results: Between 2019 and 2024, median annual OOP costs for most agents ranged from $4,000 to $13,000. In 2025, OOP costs dropped sharply to the $2,000 cap or below for all agents, while retail prices remained stable or slightly increased. Interrupted time series analysis demonstrated a significant policy-driven decline in OOP spending after 2025 across most drugs (excluding adalimumab and risankinumab due to insufficient data) with absolute reductions ranging from $3,283–$10,922 (p< 0.05)
Discussion: Implementation of the IRA's $2,000 OOP cap in 2025 was associated with a marked and immediate reduction in financial burden for high-cost IBD therapies. Our findings highlight the potential of policy interventions to improve medication affordability and support adherence for Medicare beneficiaries.
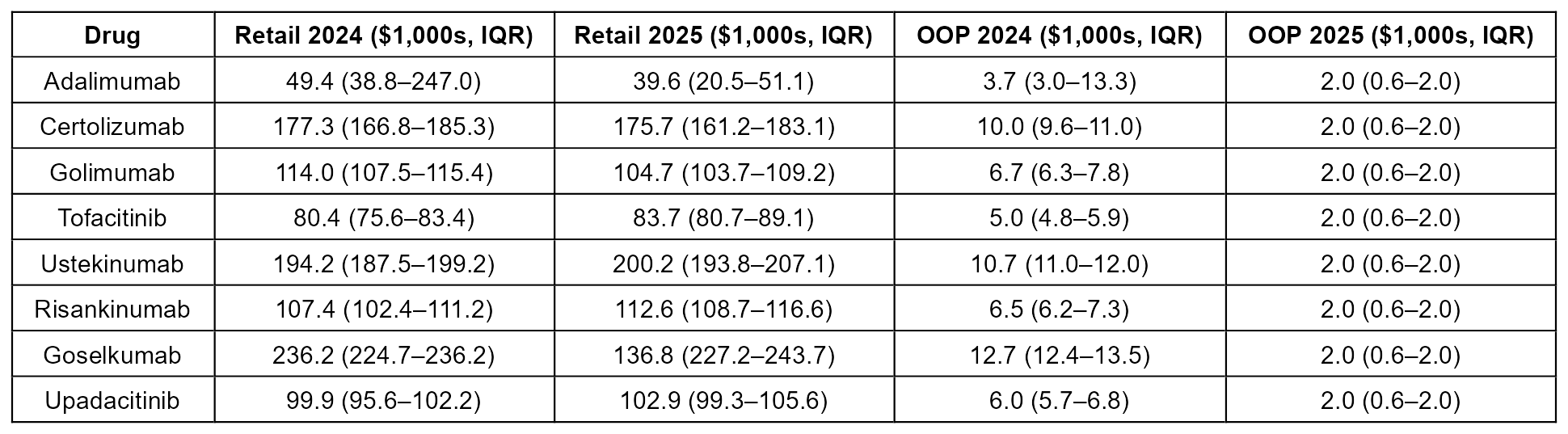
Figure: Figure 1: Interrupted Time Series Analysis of Out of Pocket costs for IBD medications (2019-2025)
Effect size and statistical significance of 2025 cost drop shown in facet titles
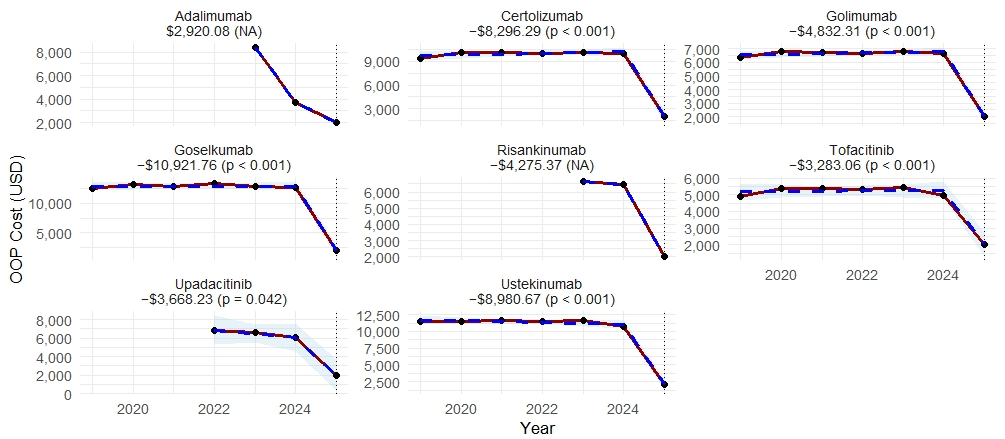
Figure: Table 1 summarizes 2024 and 2025 retail and OOP costs with interquartile ranges, reported in thousands of dollars.
Disclosures:
Mythili Menon Pathiyil indicated no relevant financial relationships.
Arvind Kumar Venkataramana Raju indicated no relevant financial relationships.
Parth Patel indicated no relevant financial relationships.
Rutwik Sharma indicated no relevant financial relationships.
Victor Chedid: Pfizer – Grant/Research Support. Takeda – Consultant.
Mythili Menon Pathiyil, MD1, Arvind Kumar Venkataramana Raju, MD1, Parth Patel, MD2, Rutwik Pradeep. Sharma, MD3, Victor Chedid, MD, MSc4. P5428 - Trends in Out-of-Pocket Spending for Drugs Under Medicare Part D in Moderate to Severe Inflammatory Bowel Disease and the Impact of the Inflation Reduction Act, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Saint Vincent Hospital, Worcester, MA; 2Ascension Saint Joseph Hospital, Chicago, IL; 3University of Michigan-Sparrow Hospital, Lansing, MI; 4Mayo Clinic, Rochester, MN
Introduction: Treatments for moderate to severe inflammatory bowel disease (IBD) include biologics or small molecules. Most are branded with limited generic substitution, resulting in high out-of-pocket (OOP) expenses that can lead to premature drug discontinuation. We aimed to assess trends in OOP costs for key IBD therapies covered under Medicare Part D and evaluate the financial impact of the Inflation Reduction Act (IRA) $2,000 OOP cap implemented in 2025.
Methods: We analyzed Medicare Part D Prescription Drug Plan Formulary and Pricing Files from Q3 (July–September) of 2019–2024 and Q1 of 2025. Eight available IBD drugs were included: adalimumab, tofacitinib, upadacitinib, ustekinumab, risankizumab, guselkumab, certolizumab and golimumab. Annual retail and OOP costs were estimated (maintenance doses for ustekinumab and risankizumab and both induction and maintenance doses for the rest) per drug using Medicare Part D benefit structures for each year. Costs were inflation-adjusted to 2025 U.S. dollars using a standardized conversion factor. Data analysis was performed in Stata 18; time trends were visualized using R.
Results: Between 2019 and 2024, median annual OOP costs for most agents ranged from $4,000 to $13,000. In 2025, OOP costs dropped sharply to the $2,000 cap or below for all agents, while retail prices remained stable or slightly increased. Interrupted time series analysis demonstrated a significant policy-driven decline in OOP spending after 2025 across most drugs (excluding adalimumab and risankinumab due to insufficient data) with absolute reductions ranging from $3,283–$10,922 (p< 0.05)
Discussion: Implementation of the IRA's $2,000 OOP cap in 2025 was associated with a marked and immediate reduction in financial burden for high-cost IBD therapies. Our findings highlight the potential of policy interventions to improve medication affordability and support adherence for Medicare beneficiaries.

Figure: Figure 1: Interrupted Time Series Analysis of Out of Pocket costs for IBD medications (2019-2025)
Effect size and statistical significance of 2025 cost drop shown in facet titles

Figure: Table 1 summarizes 2024 and 2025 retail and OOP costs with interquartile ranges, reported in thousands of dollars.
Disclosures:
Mythili Menon Pathiyil indicated no relevant financial relationships.
Arvind Kumar Venkataramana Raju indicated no relevant financial relationships.
Parth Patel indicated no relevant financial relationships.
Rutwik Sharma indicated no relevant financial relationships.
Victor Chedid: Pfizer – Grant/Research Support. Takeda – Consultant.
Mythili Menon Pathiyil, MD1, Arvind Kumar Venkataramana Raju, MD1, Parth Patel, MD2, Rutwik Pradeep. Sharma, MD3, Victor Chedid, MD, MSc4. P5428 - Trends in Out-of-Pocket Spending for Drugs Under Medicare Part D in Moderate to Severe Inflammatory Bowel Disease and the Impact of the Inflation Reduction Act, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
