Tuesday Poster Session
Category: IBD
P5426 - Real-World Impact of Risankizumab on Health-Related Quality of Life in Adults With Crohn’s Disease: Initial Results From the ASPIRE-CD Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Laurie Keefer, PhD, FACG (she/her/hers)
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Laurie Keefer, PhD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Aline Charabaty, MD, FACG8
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 3AbbVie Inc, North Chicago, IL; 4Analysis Group, Inc, Boston, MA; 5Analysis Group, Inc., Boston, MA; 6AbbVie Inc., North Chicago, IL; 7AbbVie, North Chicago, IL; 8Johns Hopkins University School of Medicine, Washington, DC
Introduction: Restoring health-related quality of life (HRQL) is an important long-term treatment target for patients with Crohn’s disease (CD). Risankizumab (RZB), an IL-23 p19 inhibitor, has shown improvements in HRQL for patients with moderate to severe CD in clinical trials. Here, we report initial results of real-world impact of RZB treatment on HRQL in patients with CD from the prospective, observational ASPIRE-CD study.
Methods: Adult patients who enrolled in the RZB patient support program in the USA were invited to participate in ASPIRE-CD. Patients were asked to complete a survey at baseline and at weeks (wks) 2, 4, and 12 after the first RZB induction dose. The survey assessed different CD-related aspects of HRQL via the Inflammatory Bowel Disease Questionnaire (IBDQ), the Short-Form 12-Item Survey (SF-12 v2) physical and mental component scores (PCS and MCS), the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-Fatigue), and the impact of CD on sexual interest/activity.
Results: Of 286 patients who received the first RZB induction dose, 241 (84.3%) completed the 12-wk survey. Those completing the wk12 survey had an average age of 46.1 years, 57.7% were female, and 74.3% had prior biologic or Janus kinase inhibitor (JAKi) exposure with 43.6% having ≥ 2 prior biologics or JAKis in their treatment history at baseline. The proportion of patients who achieved IBDQ response at wks 4 and 12 were 36.5% and 38.2%, respectively (Fig. 1A). At wk12, patients achieving IBDQ remission increased from 34.3% at baseline to 54.8% (P < .001; Fig. 1B) and the mean SF-12 PCS increased from 46.2 to 49.0 (P < .001; Fig. 1C). The mean SF-12 MCS remained stable overall and for most MCS domains through 12 wks, except for the mean social functioning domain score, which increased from 45.4 to 48.2 (P < .01). Among patients who experienced fatigue (FACIT-Fatigue score of < 40) at baseline (189/286 [66.1%]), 23.1% achieved FACIT-Fatigue response by wk12 (Fig. 2A). At wk12, patients reporting that CD “very much” negatively affected sexual interest/activity decreased from 19.8% at baseline to 12.6%; whereas those who answered “not at all” increased from 31.6% to 44.2% (P < .01; Fig. 2B).
Discussion: Initial results from the ASPIRE-CD study showed improvements in HRQL, including reduced fatigue and improved sexual interest/satisfaction, from baseline to as early as 4 wks of RZB initiation, which continued through wk12. Future data will characterize the long-term real-world experiences of these patients.
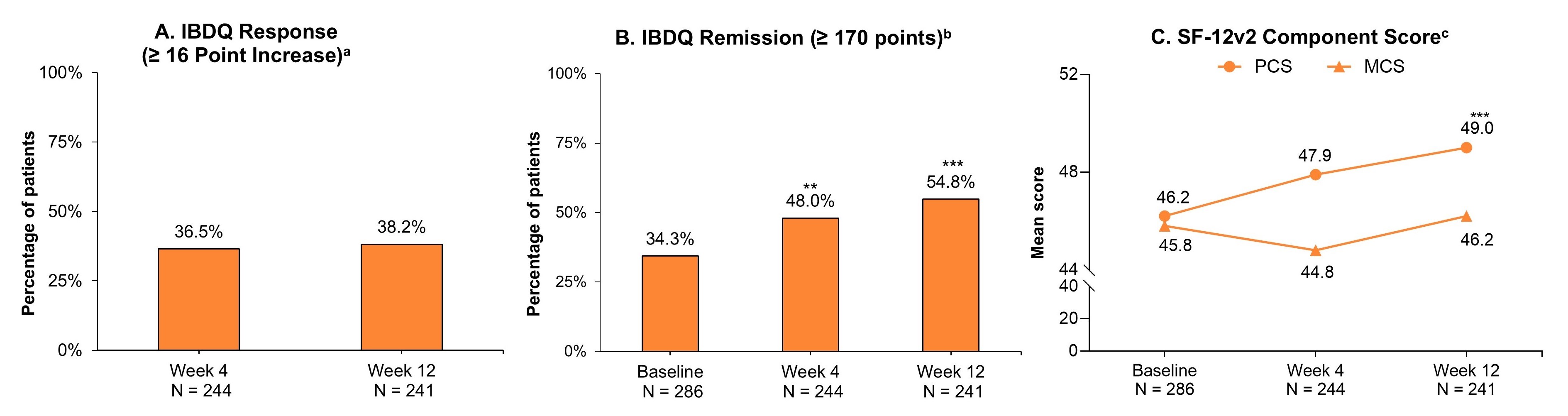
Figure: Figure 1. Patients With CD who Initiated RZB and Achieved IBDQ Response, IBDQ Remission, and SF-12v2 Component Scores.
Abbreviations: HRQL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; N, number; RZB, Risankizumab; SF-12v2, Short-Form 12-Item Survey-version 2.
* P < .05, ** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
a≥ 16 point increase in IBDQ total score (source: Aladraj H, et al. J Clin Med. 2022. 11:3743).
bIBDQ total score ≥ 170 points.
cScoring is normalized with a mean of 50 and a standard deviation of 10 in the general population where higher scores indicate better HRQL.
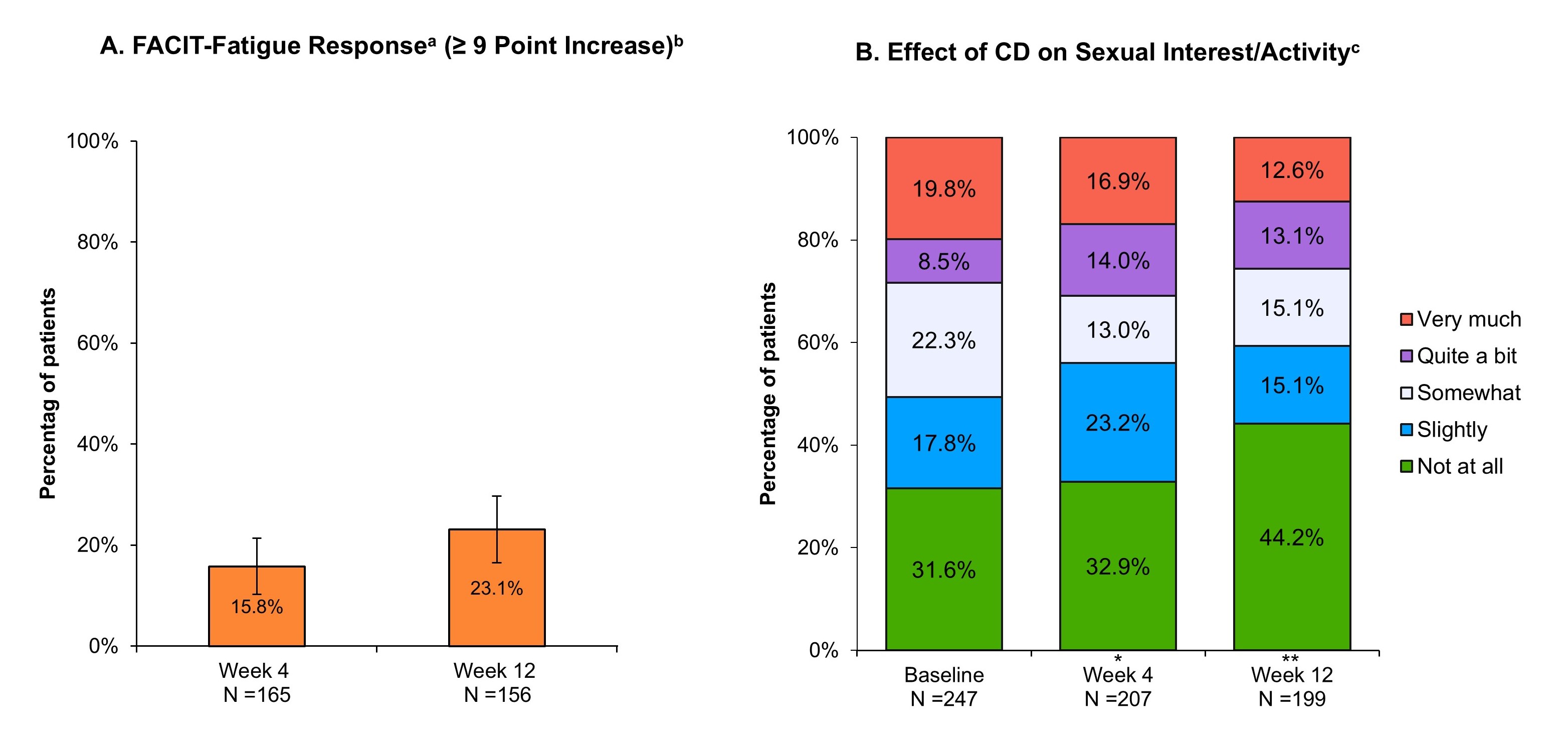
Figure: Figure 2. Patient Changes in Fatigue and Sexual Interest/Satisfaction.
Abbreviations: CD, Crohn’s disease; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; N, number; RZB, Risankizumab.
* P < .05, ** P < .01.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
aAmong patients who experienced fatigue at baseline. Fatigue at baseline is defined as patients with a FACIT-Fatigue score of < 40 at baseline (source: Louis E, et al. Dig Liver Dis. 2025. 57:707–15).
bSource: Regueiro M, et al. Qual Life Res. 2025. 34:509-521).
cNumber of respondents are those that did not select “Not applicable” for the question “In the past 4 weeks, how much has your sexual interest and/or activity been negatively affected by Crohn’s disease?”.
Disclosures:
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Julia Vishnevetsky: AbbVie Inc. – Employee, Stock Options.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Laurie Keefer, PhD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Aline Charabaty, MD, FACG8. P5426 - Real-World Impact of Risankizumab on Health-Related Quality of Life in Adults With Crohn’s Disease: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2Division of Gastroenterology & Hepatology, Houston Methodist-Weill Cornell, Houston, TX; 3AbbVie Inc, North Chicago, IL; 4Analysis Group, Inc, Boston, MA; 5Analysis Group, Inc., Boston, MA; 6AbbVie Inc., North Chicago, IL; 7AbbVie, North Chicago, IL; 8Johns Hopkins University School of Medicine, Washington, DC
Introduction: Restoring health-related quality of life (HRQL) is an important long-term treatment target for patients with Crohn’s disease (CD). Risankizumab (RZB), an IL-23 p19 inhibitor, has shown improvements in HRQL for patients with moderate to severe CD in clinical trials. Here, we report initial results of real-world impact of RZB treatment on HRQL in patients with CD from the prospective, observational ASPIRE-CD study.
Methods: Adult patients who enrolled in the RZB patient support program in the USA were invited to participate in ASPIRE-CD. Patients were asked to complete a survey at baseline and at weeks (wks) 2, 4, and 12 after the first RZB induction dose. The survey assessed different CD-related aspects of HRQL via the Inflammatory Bowel Disease Questionnaire (IBDQ), the Short-Form 12-Item Survey (SF-12 v2) physical and mental component scores (PCS and MCS), the Functional Assessment of Chronic Illness Therapy – Fatigue (FACIT-Fatigue), and the impact of CD on sexual interest/activity.
Results: Of 286 patients who received the first RZB induction dose, 241 (84.3%) completed the 12-wk survey. Those completing the wk12 survey had an average age of 46.1 years, 57.7% were female, and 74.3% had prior biologic or Janus kinase inhibitor (JAKi) exposure with 43.6% having ≥ 2 prior biologics or JAKis in their treatment history at baseline. The proportion of patients who achieved IBDQ response at wks 4 and 12 were 36.5% and 38.2%, respectively (Fig. 1A). At wk12, patients achieving IBDQ remission increased from 34.3% at baseline to 54.8% (P < .001; Fig. 1B) and the mean SF-12 PCS increased from 46.2 to 49.0 (P < .001; Fig. 1C). The mean SF-12 MCS remained stable overall and for most MCS domains through 12 wks, except for the mean social functioning domain score, which increased from 45.4 to 48.2 (P < .01). Among patients who experienced fatigue (FACIT-Fatigue score of < 40) at baseline (189/286 [66.1%]), 23.1% achieved FACIT-Fatigue response by wk12 (Fig. 2A). At wk12, patients reporting that CD “very much” negatively affected sexual interest/activity decreased from 19.8% at baseline to 12.6%; whereas those who answered “not at all” increased from 31.6% to 44.2% (P < .01; Fig. 2B).
Discussion: Initial results from the ASPIRE-CD study showed improvements in HRQL, including reduced fatigue and improved sexual interest/satisfaction, from baseline to as early as 4 wks of RZB initiation, which continued through wk12. Future data will characterize the long-term real-world experiences of these patients.

Figure: Figure 1. Patients With CD who Initiated RZB and Achieved IBDQ Response, IBDQ Remission, and SF-12v2 Component Scores.
Abbreviations: HRQL, health-related quality of life; IBDQ, Inflammatory Bowel Disease Questionnaire; N, number; RZB, Risankizumab; SF-12v2, Short-Form 12-Item Survey-version 2.
* P < .05, ** P < .01, *** P < .001.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
a≥ 16 point increase in IBDQ total score (source: Aladraj H, et al. J Clin Med. 2022. 11:3743).
bIBDQ total score ≥ 170 points.
cScoring is normalized with a mean of 50 and a standard deviation of 10 in the general population where higher scores indicate better HRQL.

Figure: Figure 2. Patient Changes in Fatigue and Sexual Interest/Satisfaction.
Abbreviations: CD, Crohn’s disease; FACIT-F, Functional Assessment of Chronic Illness Therapy – Fatigue; N, number; RZB, Risankizumab.
* P < .05, ** P < .01.
Baseline includes patients who initiated RZB with induction dosage administered by intravenous infusion. Statistical comparisons between baseline and post-treatment outcomes were conducted using Chi-square/Fisher’s exact tests for categorical variables.
aAmong patients who experienced fatigue at baseline. Fatigue at baseline is defined as patients with a FACIT-Fatigue score of < 40 at baseline (source: Louis E, et al. Dig Liver Dis. 2025. 57:707–15).
bSource: Regueiro M, et al. Qual Life Res. 2025. 34:509-521).
cNumber of respondents are those that did not select “Not applicable” for the question “In the past 4 weeks, how much has your sexual interest and/or activity been negatively affected by Crohn’s disease?”.
Disclosures:
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Bincy Abraham: Abbvie – Consultant, Speakers Bureau. Celltrion – Consultant. Eli Lilly – Consultant, Speakers Bureau. Johnson and Johnson – Consultant, Speakers Bureau. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant, Speakers Bureau.
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Julia Vishnevetsky: AbbVie Inc. – Employee, Stock Options.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Aline Charabaty: AbbVie – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Celltrion – Grant/Research Support. Eli Lilly – Advisory Committee/Board Member, Consultant. guardant health – Consultant. Janssen – Advisory Committee/Board Member, Consultant. Pfizer Inc – Advisory Committee/Board Member, Consultant. sanofi – Advisor or Review Panel Member, Consultant. scrubs & heels foundation – co-founder. Takeda – Advisory Committee/Board Member, Consultant.
Laurie Keefer, PhD, FACG1, Bincy Abraham, MD, MS, FACG2, Jenny M. Griffith, PharmD3, Min Yang, MD4, Erin E. Cook, PhD5, Javier Zambrano, MD6, Julia Vishnevetsky, MPH7, Bruno Martins, PhD5, Aline Charabaty, MD, FACG8. P5426 - Real-World Impact of Risankizumab on Health-Related Quality of Life in Adults With Crohn’s Disease: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

