Tuesday Poster Session
Category: IBD
P5425 - Treatment Satisfaction and Patients’ Experiences with Risankizumab On-Body Injector Among Adults With Crohn's Disease in Clinical Practice: Initial Results From the ASPIRE-CD Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Laurie Keefer, PhD, FACG (she/her/hers)
Icahn School of Medicine at Mount Sinai
New York, NY
Presenting Author(s)
Laurie Keefer, PhD, FACG1, Jenny M. Griffith, PharmD2, Min Yang, MD3, Bruno Martins, PhD4, Javier Zambrano, MD5, Erin E. Cook, PhD4, Namita Joshi, PhD6, Brooke Kim, RN6, Aline Charabaty, MD, FACG7
1Icahn School of Medicine at Mount Sinai, New York, NY; 2AbbVie Inc, North Chicago, IL; 3Analysis Group, Inc, Boston, MA; 4Analysis Group, Inc., Boston, MA; 5AbbVie Inc., North Chicago, IL; 6AbbVie, North Chicago, IL; 7Division of Gastroenterology and Hepatology, Johns Hopkins School of Medicine, Washington, DC
Introduction: Risankizumab (RZB) has been approved to treat adults with moderately to severely active Crohn’s disease (CD). Following intravenous RZB induction, patients continue with RZB maintenance therapy starting at Week 12 via subcutaneous injections using an On-Body Injector (OBI). Here we report the initial experiences and satisfaction by Week 12 in patients enrolled in ASPIRE-CD, an ongoing, observational, prospective, longitudinal patient survey study.
Methods: Adult patients with CD prescribed with RZB and enrolled in the RZB patient support program (PSP) in the US were invited to participate in ASPIRE-CD. Patients completed surveys before (baseline) and at Weeks 2, 4, 12, and every 8 weeks after RZB initiation. Satisfaction with current CD treatments was evaluated using a 7-response Likert scale (from “extremely satisfied” to “extremely dissatisfied”). At initiation of OBI (Week 12), ease of learning and ease of using the OBI were assessed with a 5-response Likert scale (“very easy” to “very difficult”), as well as patients’ satisfaction with the OBI and PSP (5-response Likert scale: “very satisfied” to “very dissatisfied”).
Results: The ASPIRE-CD study included 286 patients who initiated RZB intravenously (female: 57.0%; mean [SD] age: 45.7 [14.8] years; disease duration: 14.2 [14.0] years; bio-/Janus kinase inhibitor-experienced: 79.0%), and 241 (84.3%) completed the Week 12 survey. At baseline, half of the patients reported being at least somewhat satisfied with their CD treatments; by Week 12, most (77.6%) were at least somewhat satisfied (p< 0.01) (Fig. 1). At Week 12, 186 patients had used the OBI and the majority reported that the injection device was easy or very easy to learn (84.9%) and to use (87.1%) (Fig. 2). Majority of these patients (91.9%) were satisfied or very satisfied with the injection device. A large proportion of patients reported using the PSP services by Week 12 (87.6%); the overall satisfaction was high among them, with 92.7% being somewhat or very satisfied with the PSP.
Discussion: Initial results from the ASPIRE-CD study at the time of the first subcutaneous injection showed that the OBI device was easy to learn and to use and patients were satisfied with the device and the PSP. Overall, most patients were satisfied with their CD treatments after the initial 12 weeks post RZB initiation. While the data on this real-world study is still maturing, future data will characterize the long-term experiences of these patients.
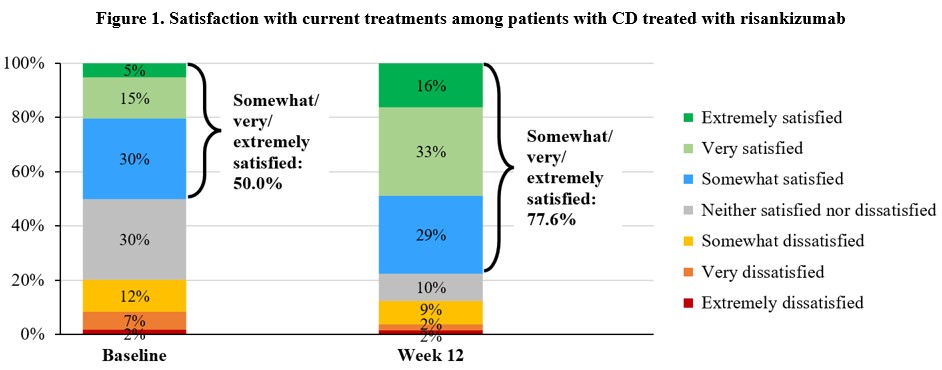
Figure: Notes:
[1] Satisfaction with current treatments was collected at baseline and week 12 using a single-item Likert scale (“How satisfied are you with your current treatment(s) for Crohn’s disease?”) with 7 response options.
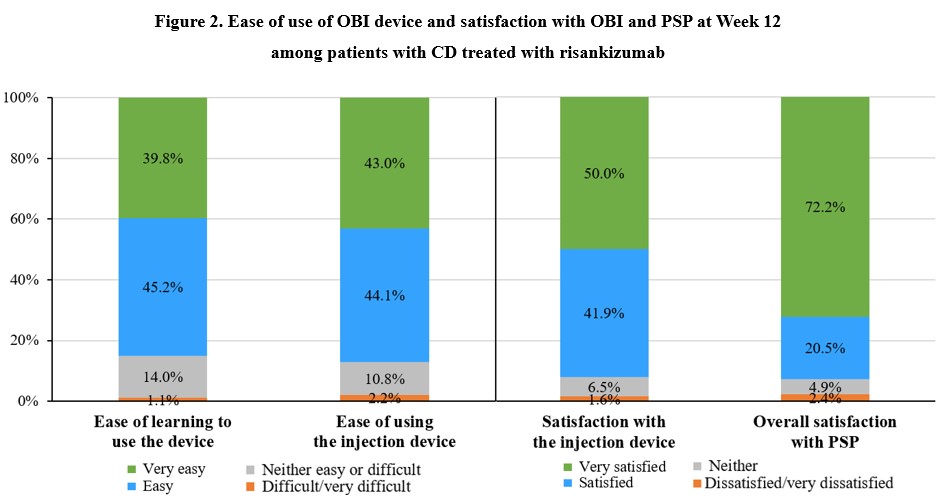
Figure: Notes:
[1] Figure summarizes responses of participants at Week 12.
[2] Ease of learning to use the device was assessed with the question “How easy is it to learn to use the device for injection?”; ease of using the injection device was assessed with the question “Overall, how easy is it to use the injection device?”; satisfaction with the injection device was assessed with the question “Overall, how satisfied are you with the injection device?”; overall satisfaction with PSP was assessed with the question “Overall, how satisfied are you with the Skyrizi Complete patient support program?”.
Disclosures:
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Namita Joshi: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Brooke Kim: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Aline Charabaty: Janssen, Takeda, AbbVie, and Pfizer – Advisor or Review Panel Member, Consultant.
Laurie Keefer, PhD, FACG1, Jenny M. Griffith, PharmD2, Min Yang, MD3, Bruno Martins, PhD4, Javier Zambrano, MD5, Erin E. Cook, PhD4, Namita Joshi, PhD6, Brooke Kim, RN6, Aline Charabaty, MD, FACG7. P5425 - Treatment Satisfaction and Patients’ Experiences with Risankizumab On-Body Injector Among Adults With Crohn's Disease in Clinical Practice: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Icahn School of Medicine at Mount Sinai, New York, NY; 2AbbVie Inc, North Chicago, IL; 3Analysis Group, Inc, Boston, MA; 4Analysis Group, Inc., Boston, MA; 5AbbVie Inc., North Chicago, IL; 6AbbVie, North Chicago, IL; 7Division of Gastroenterology and Hepatology, Johns Hopkins School of Medicine, Washington, DC
Introduction: Risankizumab (RZB) has been approved to treat adults with moderately to severely active Crohn’s disease (CD). Following intravenous RZB induction, patients continue with RZB maintenance therapy starting at Week 12 via subcutaneous injections using an On-Body Injector (OBI). Here we report the initial experiences and satisfaction by Week 12 in patients enrolled in ASPIRE-CD, an ongoing, observational, prospective, longitudinal patient survey study.
Methods: Adult patients with CD prescribed with RZB and enrolled in the RZB patient support program (PSP) in the US were invited to participate in ASPIRE-CD. Patients completed surveys before (baseline) and at Weeks 2, 4, 12, and every 8 weeks after RZB initiation. Satisfaction with current CD treatments was evaluated using a 7-response Likert scale (from “extremely satisfied” to “extremely dissatisfied”). At initiation of OBI (Week 12), ease of learning and ease of using the OBI were assessed with a 5-response Likert scale (“very easy” to “very difficult”), as well as patients’ satisfaction with the OBI and PSP (5-response Likert scale: “very satisfied” to “very dissatisfied”).
Results: The ASPIRE-CD study included 286 patients who initiated RZB intravenously (female: 57.0%; mean [SD] age: 45.7 [14.8] years; disease duration: 14.2 [14.0] years; bio-/Janus kinase inhibitor-experienced: 79.0%), and 241 (84.3%) completed the Week 12 survey. At baseline, half of the patients reported being at least somewhat satisfied with their CD treatments; by Week 12, most (77.6%) were at least somewhat satisfied (p< 0.01) (Fig. 1). At Week 12, 186 patients had used the OBI and the majority reported that the injection device was easy or very easy to learn (84.9%) and to use (87.1%) (Fig. 2). Majority of these patients (91.9%) were satisfied or very satisfied with the injection device. A large proportion of patients reported using the PSP services by Week 12 (87.6%); the overall satisfaction was high among them, with 92.7% being somewhat or very satisfied with the PSP.
Discussion: Initial results from the ASPIRE-CD study at the time of the first subcutaneous injection showed that the OBI device was easy to learn and to use and patients were satisfied with the device and the PSP. Overall, most patients were satisfied with their CD treatments after the initial 12 weeks post RZB initiation. While the data on this real-world study is still maturing, future data will characterize the long-term experiences of these patients.

Figure: Notes:
[1] Satisfaction with current treatments was collected at baseline and week 12 using a single-item Likert scale (“How satisfied are you with your current treatment(s) for Crohn’s disease?”) with 7 response options.

Figure: Notes:
[1] Figure summarizes responses of participants at Week 12.
[2] Ease of learning to use the device was assessed with the question “How easy is it to learn to use the device for injection?”; ease of using the injection device was assessed with the question “Overall, how easy is it to use the injection device?”; satisfaction with the injection device was assessed with the question “Overall, how satisfied are you with the injection device?”; overall satisfaction with PSP was assessed with the question “Overall, how satisfied are you with the Skyrizi Complete patient support program?”.
Disclosures:
Laurie Keefer: AbbVie – Consultant. Ardelyx – Consultant. Eli Lilly – Consultant. Janssen – Consultant. Pfizer – Consultant. Reckitt Health – Consultant. Trellus Health – Owner/Ownership Interest, Stock-publicly held company(excluding mutual/index funds).
Jenny M. Griffith: AbbVie – Employee, Stock Options.
Min Yang: Analysis Group, AbbVie Inc. – Consultant.
Bruno Martins: Analysis Group, AbbVie Inc. – Consultant.
Javier Zambrano: AbbVie Inc. – Employee, Stock Options.
Erin Cook: Analysis Group, AbbVie Inc. – Consultant.
Namita Joshi: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Brooke Kim: AbbVie – Employee, Stock-publicly held company(excluding mutual/index funds).
Aline Charabaty: Janssen, Takeda, AbbVie, and Pfizer – Advisor or Review Panel Member, Consultant.
Laurie Keefer, PhD, FACG1, Jenny M. Griffith, PharmD2, Min Yang, MD3, Bruno Martins, PhD4, Javier Zambrano, MD5, Erin E. Cook, PhD4, Namita Joshi, PhD6, Brooke Kim, RN6, Aline Charabaty, MD, FACG7. P5425 - Treatment Satisfaction and Patients’ Experiences with Risankizumab On-Body Injector Among Adults With Crohn's Disease in Clinical Practice: Initial Results From the ASPIRE-CD Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
