Tuesday Poster Session
Category: IBD
P5415 - Allogeneic Mesenchymal Stem Cells in the Treatment of Perianal Fistula in Crohn’s Disease: A Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- MA
Mageda Al Areqi, MD
Raritan Bay Medical Center
Perth Amboy, NJ
Presenting Author(s)
Mohammed S. Beshr, MBBS1, Rana H. Shembesh, MBBCh2, Abdallah Khashan, MD3, Mageda Al Areqi, MD4, Walid Guerguer, MD5, Haron A. Al-Badawi, MBBS6, Anas A. Al-Amrani, MBBS6, Arwi Kara, MD7, Ahmed Abraheem, MD8, Muhammed Elhadi, MD9
1Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 2Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 3Capital Health Regional Medical Center, Trenton, NJ; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5Faculty of medicine, University of Algiers 1, El Biar, Alger, Algeria; 6Faculty of Medicine, Sana'a University, Sana'a, San'a', Yemen; 7Faculty of Medicine, University Of Benghazi, Benghazi, Benghazi, Libya; 8Faculty of medicine, university of al-azhar, Cairo, Al Qahirah, Egypt; 9College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: Perianal fistula can be a complex problem and difficult to treat in Crohn's disease. Mesenchymal stem cells have shown promise in some recent trials. They are derived from the adipose tissue or bone marrow. In this paper, we aim to evaluate their ability to achieve clinical remission and safety.
Methods: On February 28, 2025 a data search was conducted using PubMed, Scopus, and the Cochrane Library. We included only human clinical trials that used mesenchymal stem cells for the treatment of perianal abscess in Crohn’s disease and had a control group. Our studied outcomes were clinical remission, defined as the complete resolution of the fistula assessed clinically and via MRI, and adverse events such as perianal abscess, proctalgia (anal pain), and the incidence of serious adverse events. We used the odds ratio with a 95% confidence interval, and a random-effects model was applied.
Results: Out of 349 articles we screened, only 6 were eligible for inclusion and analysis. All of the studies were placebo controlled. In our analysis, clinical remission was significantly higher in the intervention group with an odds ratio of 2.17 [CI: 1.54 to 3.04, p < 0.001]. There was no significant difference in terms of the incidence of perianal abscess, it had an odds ratio of 1.07 [CI: 0.68 to 1.70, p = 0.77]. Similarly, for proctalgia (anal pain), there was no significant difference between the groups, with an odds ratio of 1.19 [CI: 0.46 to 3.10, p = 0.72]. Finally, there was no difference in terms of the incidence of serious adverse events [OR: 1.28, CI: 0.78 to 2.10, p = 0.34].
Discussion: Our paper suggests that mesenchymal stem cells may have a role in the treatment of perineal fistula in Crohn's disease patients. Their use was associated with higher clinical remission rates. Large controlled clinical trials are needed to confirm their efficacy and establish their safety profile.
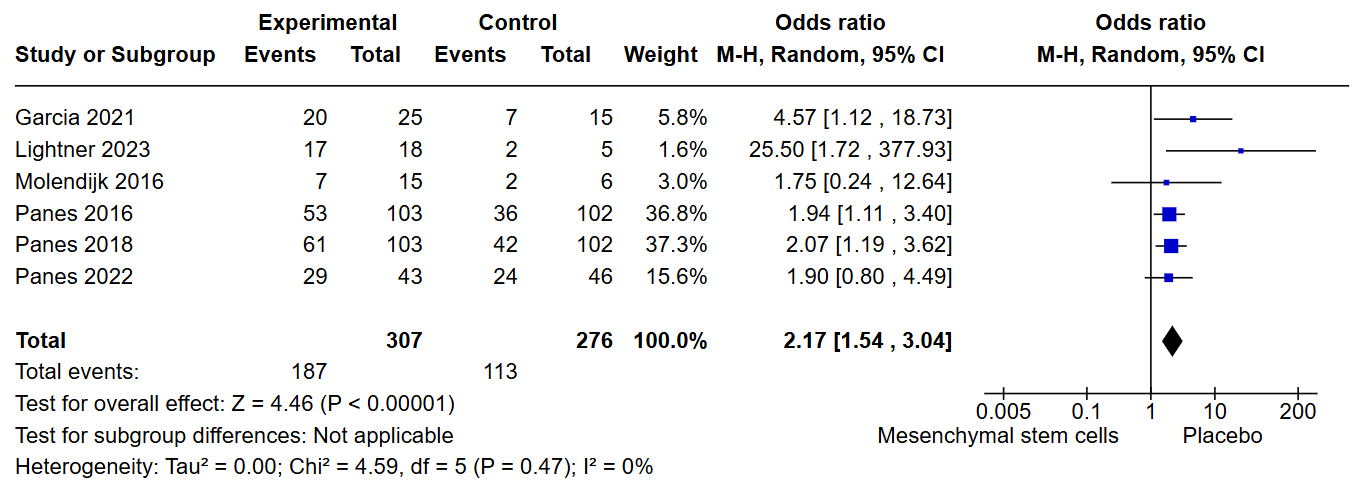
Figure: Figure 1: Clinical Remission
Figure: Figure 2: perineal Abscess
Disclosures:
Mohammed Beshr indicated no relevant financial relationships.
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Walid Guerguer indicated no relevant financial relationships.
Haron Al-Badawi indicated no relevant financial relationships.
Anas Al-Amrani indicated no relevant financial relationships.
Arwi Kara indicated no relevant financial relationships.
Ahmed Abraheem indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Mohammed S. Beshr, MBBS1, Rana H. Shembesh, MBBCh2, Abdallah Khashan, MD3, Mageda Al Areqi, MD4, Walid Guerguer, MD5, Haron A. Al-Badawi, MBBS6, Anas A. Al-Amrani, MBBS6, Arwi Kara, MD7, Ahmed Abraheem, MD8, Muhammed Elhadi, MD9. P5415 - Allogeneic Mesenchymal Stem Cells in the Treatment of Perianal Fistula in Crohn’s Disease: A Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Sana’a University, Faculty of Medicine and Health Sciences, Sana'a, Hadramawt, Yemen; 2Libyan International Medical University, Faculty of Medicine, Benghazi, Benghazi, Libya; 3Capital Health Regional Medical Center, Trenton, NJ; 4Raritan Bay Medical Center, Perth Amboy, NJ; 5Faculty of medicine, University of Algiers 1, El Biar, Alger, Algeria; 6Faculty of Medicine, Sana'a University, Sana'a, San'a', Yemen; 7Faculty of Medicine, University Of Benghazi, Benghazi, Benghazi, Libya; 8Faculty of medicine, university of al-azhar, Cairo, Al Qahirah, Egypt; 9College of Medicine, Korea University, Seongbuk, Seoul-t'ukpyolsi, Republic of Korea
Introduction: Perianal fistula can be a complex problem and difficult to treat in Crohn's disease. Mesenchymal stem cells have shown promise in some recent trials. They are derived from the adipose tissue or bone marrow. In this paper, we aim to evaluate their ability to achieve clinical remission and safety.
Methods: On February 28, 2025 a data search was conducted using PubMed, Scopus, and the Cochrane Library. We included only human clinical trials that used mesenchymal stem cells for the treatment of perianal abscess in Crohn’s disease and had a control group. Our studied outcomes were clinical remission, defined as the complete resolution of the fistula assessed clinically and via MRI, and adverse events such as perianal abscess, proctalgia (anal pain), and the incidence of serious adverse events. We used the odds ratio with a 95% confidence interval, and a random-effects model was applied.
Results: Out of 349 articles we screened, only 6 were eligible for inclusion and analysis. All of the studies were placebo controlled. In our analysis, clinical remission was significantly higher in the intervention group with an odds ratio of 2.17 [CI: 1.54 to 3.04, p < 0.001]. There was no significant difference in terms of the incidence of perianal abscess, it had an odds ratio of 1.07 [CI: 0.68 to 1.70, p = 0.77]. Similarly, for proctalgia (anal pain), there was no significant difference between the groups, with an odds ratio of 1.19 [CI: 0.46 to 3.10, p = 0.72]. Finally, there was no difference in terms of the incidence of serious adverse events [OR: 1.28, CI: 0.78 to 2.10, p = 0.34].
Discussion: Our paper suggests that mesenchymal stem cells may have a role in the treatment of perineal fistula in Crohn's disease patients. Their use was associated with higher clinical remission rates. Large controlled clinical trials are needed to confirm their efficacy and establish their safety profile.

Figure: Figure 1: Clinical Remission
Figure: Figure 2: perineal Abscess
Disclosures:
Mohammed Beshr indicated no relevant financial relationships.
Rana Shembesh indicated no relevant financial relationships.
Abdallah Khashan indicated no relevant financial relationships.
Mageda Al Areqi indicated no relevant financial relationships.
Walid Guerguer indicated no relevant financial relationships.
Haron Al-Badawi indicated no relevant financial relationships.
Anas Al-Amrani indicated no relevant financial relationships.
Arwi Kara indicated no relevant financial relationships.
Ahmed Abraheem indicated no relevant financial relationships.
Muhammed Elhadi indicated no relevant financial relationships.
Mohammed S. Beshr, MBBS1, Rana H. Shembesh, MBBCh2, Abdallah Khashan, MD3, Mageda Al Areqi, MD4, Walid Guerguer, MD5, Haron A. Al-Badawi, MBBS6, Anas A. Al-Amrani, MBBS6, Arwi Kara, MD7, Ahmed Abraheem, MD8, Muhammed Elhadi, MD9. P5415 - Allogeneic Mesenchymal Stem Cells in the Treatment of Perianal Fistula in Crohn’s Disease: A Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
