Tuesday Poster Session
Category: IBD
P5492 - Endoscopic Remission Rates With Risankizumab Following Treatment Failure With Ustekinumab
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Natalia A. Filipek, MD (she/her/hers)
University of Washington Medical Center
Presenting Author(s)
Natalia A. Filipek, MD1, Kindra Clark-Snustad, DNP1, Kendra Kamp, PhD, RN2, Jason Harper, MD1, Mitra Barahimi, MD1, Allison Fairbanks, ARNP, MPH1, Nina Saxena, MD1, Scott Lee, MD1, Jeffrey Jacobs, MD1
1University of Washington Medical Center, Seattle, WA; 2University of Washington, Seattle, WA
Introduction: Risankizumab demonstrated superior efficacy in achieving endoscopic remission in patients with Crohn’s disease (CD). What remains uncertain is how CD refractory to ustekinumab does with subsequent administration of risankizumab. Our study presents endoscopic and clinical data following the use of risankizumab in CD patients who previously failed ustekinumab.
Methods: A retrospective analysis was performed on CD patients who were treated with ustekinumab followed by risankizumab given inadequate response. Patients were ≥ 18 years of age. Endoscopic and clinical data was collected with risankizumab administration. Clinical response was characterized by ≥ 3 point decrease in the Harvey-Bradshaw Index (HBI) and remission HBI ≤ 4. Endoscopic improvement was defined as ≥ 50% decrease in the simple endoscopic score (SES-CD) and endoscopic remission as SES-CD ≤ 3. Corticosteroid administration was analyzed.
Results: We report on 45 patients with CD who underwent treatment with risankizumab following ustekinumab failure. Median age was 38 years, mean CD duration was 17 years, and 82% of patients previously used >2 advanced therapies.
Indication to transition from ustekinumab to risankizumab was: non-response (37%) and inadequate response (63%). Mean risankizumab treatment time was 6.7 months.
Following risankizumab therapy, 44.4% (n= 4/9) had clinical response and 22.2% (n=2/9) achieved steroid free clinical remission. Of patients with active endoscopic disease, 24 had available endoscopic results following risankizumab therapy. In this cohort, 33.3% (n=8/24) had endoscopic improvement and 20.8% (n=5/24) achieved endoscopic remission. Mean SES-CD decreased from 16.4 (SD 9.7) to 10.0 (SD 8.7) with statistical significance following risankizumab therapy (p=0.02). While 0% (n=0/8) of patients with non-response to ustekinumab had endoscopic improvement following risankizumab, 46.2% (n=6/13) with inadequate response and 66.7% (n=2/3) with loss of response to ustekinumab had endoscopic improvement. CRP, albumin, and anemia normalization was seen in >60% of patients.
Discussion: Risankizumab administration resulted in endoscopic improvement and captured endoscopic remission in a subset of patients with long-standing, refractory CD after ustekinumab failure. While the number of analyzed patients is small, primary non-response to ustekinumab appears to predict poor response to risankizumab. Further research is needed to understand if this group of patients should consider risankizumab as alternate therapy.
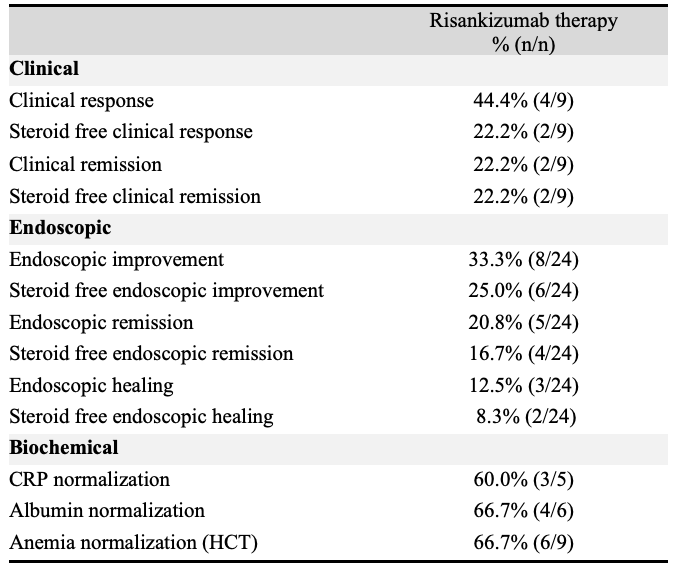
Figure: Table 1: Clinical, endoscopic, and biochemical outcomes after risankizumab therapy.
Caption - Endpoint definitions: active clinical disease (baseline HBI ≥5); clinical response (≥3-point decrease in HBI); clinical remission (HBI ≤4); active endoscopic disease (baseline SESCD ≥6, or ≥4 for isolated ileal disease); endoscopic improvement (≥50% decrease in SES-CD); endoscopic remission (SESCD ≤3); and ulcer-free endoscopy (SESCD=0). Steroid free outcomes were assessed as the outcome in the absence of oral steroids at that time point. Biochemical remission: resolution of lab abnormality (e.g. CRP, hematocrit (HCT), albumin).
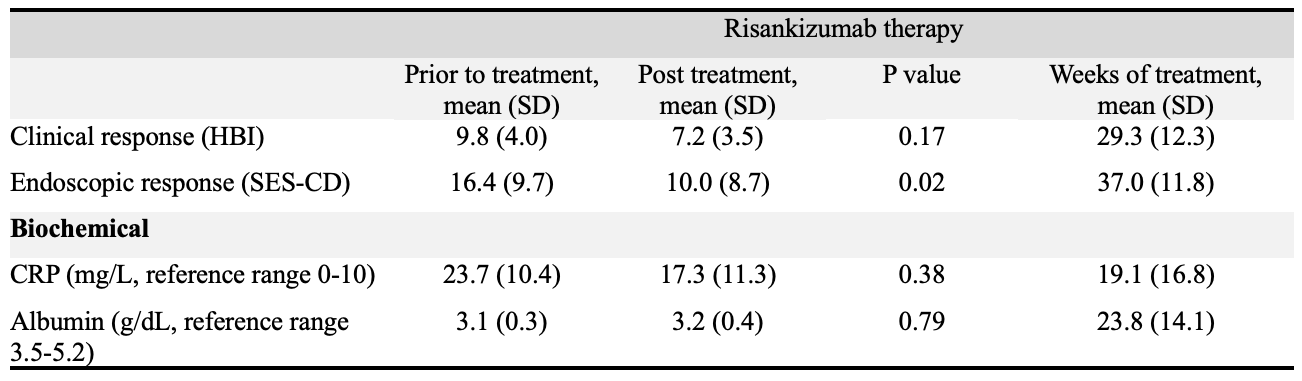
Figure: Table 2: Mean clinical disease activity score, endoscopic disease activity score, and biochemical values prior to and after risankizumab treatment.
Caption - HBI: Harvey-Bradshaw Index, SES-CD: Simple Endoscopic Score Crohn’s Disease, CRP: C-reactive protein
Disclosures:
Natalia Filipek indicated no relevant financial relationships.
Kindra Clark-Snustad: AbbVie Pharmaceuticals – Consultant. Janssen Pharmaceuticals, Inc. – Consultant. Pfizer Pharmaceuticals, Inc. – Consultant.
Kendra Kamp indicated no relevant financial relationships.
Jason Harper indicated no relevant financial relationships.
Mitra Barahimi indicated no relevant financial relationships.
Allison Fairbanks indicated no relevant financial relationships.
Nina Saxena indicated no relevant financial relationships.
Scott Lee: AbbVie Pharmaceuticals – Grant/Research Support. Abivax – Grant/Research Support. Applied Molecular Transport Inc. – Consultant. Boehringer Ingelheim Pharmaceuticals, Inc – Consultant, Grant/Research Support. Bristol-Myers Squibb – Consultant, Grant/Research Support. Celltrion, Inc. – Consultant. Eli Lilly and Company – Consultant, Grant/Research Support. Janssen Pharmaceuticals, Inc. – Consultant, Grant/Research Support. Merck, AnaptysBio, Inc – Grant/Research Support. Pfizer Pharmaceuticals, Inc. – Consultant. Protagonist – Consultant.
Jeffrey Jacobs: Iterative Health – Consultant.
Natalia A. Filipek, MD1, Kindra Clark-Snustad, DNP1, Kendra Kamp, PhD, RN2, Jason Harper, MD1, Mitra Barahimi, MD1, Allison Fairbanks, ARNP, MPH1, Nina Saxena, MD1, Scott Lee, MD1, Jeffrey Jacobs, MD1. P5492 - Endoscopic Remission Rates With Risankizumab Following Treatment Failure With Ustekinumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Washington Medical Center, Seattle, WA; 2University of Washington, Seattle, WA
Introduction: Risankizumab demonstrated superior efficacy in achieving endoscopic remission in patients with Crohn’s disease (CD). What remains uncertain is how CD refractory to ustekinumab does with subsequent administration of risankizumab. Our study presents endoscopic and clinical data following the use of risankizumab in CD patients who previously failed ustekinumab.
Methods: A retrospective analysis was performed on CD patients who were treated with ustekinumab followed by risankizumab given inadequate response. Patients were ≥ 18 years of age. Endoscopic and clinical data was collected with risankizumab administration. Clinical response was characterized by ≥ 3 point decrease in the Harvey-Bradshaw Index (HBI) and remission HBI ≤ 4. Endoscopic improvement was defined as ≥ 50% decrease in the simple endoscopic score (SES-CD) and endoscopic remission as SES-CD ≤ 3. Corticosteroid administration was analyzed.
Results: We report on 45 patients with CD who underwent treatment with risankizumab following ustekinumab failure. Median age was 38 years, mean CD duration was 17 years, and 82% of patients previously used >2 advanced therapies.
Indication to transition from ustekinumab to risankizumab was: non-response (37%) and inadequate response (63%). Mean risankizumab treatment time was 6.7 months.
Following risankizumab therapy, 44.4% (n= 4/9) had clinical response and 22.2% (n=2/9) achieved steroid free clinical remission. Of patients with active endoscopic disease, 24 had available endoscopic results following risankizumab therapy. In this cohort, 33.3% (n=8/24) had endoscopic improvement and 20.8% (n=5/24) achieved endoscopic remission. Mean SES-CD decreased from 16.4 (SD 9.7) to 10.0 (SD 8.7) with statistical significance following risankizumab therapy (p=0.02). While 0% (n=0/8) of patients with non-response to ustekinumab had endoscopic improvement following risankizumab, 46.2% (n=6/13) with inadequate response and 66.7% (n=2/3) with loss of response to ustekinumab had endoscopic improvement. CRP, albumin, and anemia normalization was seen in >60% of patients.
Discussion: Risankizumab administration resulted in endoscopic improvement and captured endoscopic remission in a subset of patients with long-standing, refractory CD after ustekinumab failure. While the number of analyzed patients is small, primary non-response to ustekinumab appears to predict poor response to risankizumab. Further research is needed to understand if this group of patients should consider risankizumab as alternate therapy.

Figure: Table 1: Clinical, endoscopic, and biochemical outcomes after risankizumab therapy.
Caption - Endpoint definitions: active clinical disease (baseline HBI ≥5); clinical response (≥3-point decrease in HBI); clinical remission (HBI ≤4); active endoscopic disease (baseline SESCD ≥6, or ≥4 for isolated ileal disease); endoscopic improvement (≥50% decrease in SES-CD); endoscopic remission (SESCD ≤3); and ulcer-free endoscopy (SESCD=0). Steroid free outcomes were assessed as the outcome in the absence of oral steroids at that time point. Biochemical remission: resolution of lab abnormality (e.g. CRP, hematocrit (HCT), albumin).

Figure: Table 2: Mean clinical disease activity score, endoscopic disease activity score, and biochemical values prior to and after risankizumab treatment.
Caption - HBI: Harvey-Bradshaw Index, SES-CD: Simple Endoscopic Score Crohn’s Disease, CRP: C-reactive protein
Disclosures:
Natalia Filipek indicated no relevant financial relationships.
Kindra Clark-Snustad: AbbVie Pharmaceuticals – Consultant. Janssen Pharmaceuticals, Inc. – Consultant. Pfizer Pharmaceuticals, Inc. – Consultant.
Kendra Kamp indicated no relevant financial relationships.
Jason Harper indicated no relevant financial relationships.
Mitra Barahimi indicated no relevant financial relationships.
Allison Fairbanks indicated no relevant financial relationships.
Nina Saxena indicated no relevant financial relationships.
Scott Lee: AbbVie Pharmaceuticals – Grant/Research Support. Abivax – Grant/Research Support. Applied Molecular Transport Inc. – Consultant. Boehringer Ingelheim Pharmaceuticals, Inc – Consultant, Grant/Research Support. Bristol-Myers Squibb – Consultant, Grant/Research Support. Celltrion, Inc. – Consultant. Eli Lilly and Company – Consultant, Grant/Research Support. Janssen Pharmaceuticals, Inc. – Consultant, Grant/Research Support. Merck, AnaptysBio, Inc – Grant/Research Support. Pfizer Pharmaceuticals, Inc. – Consultant. Protagonist – Consultant.
Jeffrey Jacobs: Iterative Health – Consultant.
Natalia A. Filipek, MD1, Kindra Clark-Snustad, DNP1, Kendra Kamp, PhD, RN2, Jason Harper, MD1, Mitra Barahimi, MD1, Allison Fairbanks, ARNP, MPH1, Nina Saxena, MD1, Scott Lee, MD1, Jeffrey Jacobs, MD1. P5492 - Endoscopic Remission Rates With Risankizumab Following Treatment Failure With Ustekinumab, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

