Tuesday Poster Session
Category: IBD
P5491 - Safety, Tolerability, and Efficacy of PL8177 in Adults With Active Ulcerative Colitis: Results From a Phase 2a, Randomized, Placebo-Controlled Study
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Dana J. Lukin, MD, PhD, FACG
Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine
New York, NY
Presenting Author(s)
Award: ACG Presidential Poster Award
Dana J. Lukin, MD, PhD, FACG1, Robert Jordan, BS2, Nazish Huq, MS2, Carl Spana, PhD2, Paul Kayne, PhD2
1Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY; 2Palatin Technologies, Inc., Cranbury, NJ
Introduction: PL8177 is a synthetic, selective melanocortin receptor 1 (MC1R) agonist. The oral formulation contains polymers to inhibit degradation in the upper gastrointestinal (GI) tract and optimize lower GI release. In a rat model of ulcerative colitis (UC), PL8177 reduced colonic macroscopic damage, fecal occult blood, and colon weight and improved stool consistency. Here, we present clinical results from a phase 2a, double-blind, randomized, placebo-controlled, parallel group study of the safety, tolerability, and efficacy of PL8177 in adults with UC.
Methods: Participants aged 18-75 years with active, mild-to-moderate UC received PL8177 (20 mg) or placebo (randomized 3:1) once daily for 56 days. Endoscopic examination of the colon with biopsy was performed at baseline and at day 56. Blood and/or fecal samples were collected on days 1, 15, 29, and 56. Clinical response, clinical remission, and symptomatic remission were determined using the modified Mayo score (endoscopy, rectal bleeding, stool frequency) (Table). The primary endpoint was an endoscopic subscore of 0 or 1 (modified to exclude friability). Adverse events (AEs) were collected.
Results: A total of 64 participants were screened and 12 participants (PL8177, n=9; placebo, n=3) were randomized. Mean age was 47.1 years (range, 24-75) and 50.0% were female. A total of 12 AEs in 4 participants and 6 AEs in 1 participant were reported in PL8177 and placebo groups, respectively. All AEs were deemed not related or unlikely related to treatment. A clinical response was achieved in 7 of 9 participants (78%) in the PL8177 group compared with 1 of 3 (33%) in the placebo group (Figure). Clinical remission was achieved by 3 of 9 participants (33%) in the PL8177 group (vs none with placebo), while symptomatic remission was achieved by 5 of 9 (56%) with PL8177 (vs 1 of 3 [33%] with placebo). The planned primary endpoint was achieved by 3 of 9 participants (33%) with PL8177 and 1 of 3 (33%) with placebo.
Discussion: There is an unmet need for pharmacologic treatments that target novel, pathologic mechanisms of UC and/or inflammatory bowel disease. In this proof-of-principle phase 2a study, PL8177 provides broad efficacy at 8 weeks across key endpoints defined by the FDA (clinical remission and response) and also in symptomatic remission. The efficacy and excellent tolerability represent a highly differentiated profile and potential alternative to current immunosuppressive and steroid treatments.
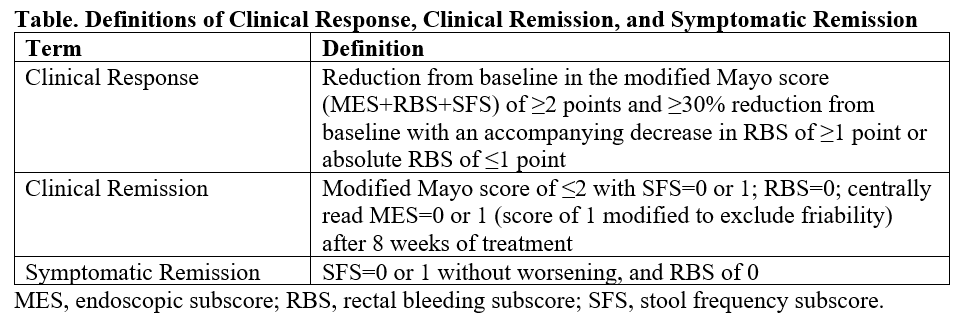
Figure: Table. Definitions of Clinical Response, Clinical Remission, and Symptomatic Remission
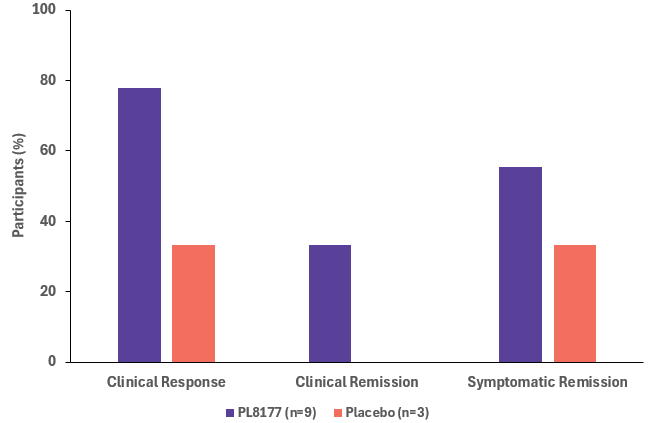
Figure: Figure. Proportion of Participants to Achieve a Clinical Response, Clinical Remission, or Symptomatic Remission
Disclosures:
Dana Lukin: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Robert Jordan: Palatin Technologies, Inc. – Employee.
Nazish Huq: Palatin Technologies, Inc. – Employee.
Carl Spana: Palatin Technologies, Inc. – Employee.
Paul Kayne: Palatin Technologies, Inc. – Employee.
Dana J. Lukin, MD, PhD, FACG1, Robert Jordan, BS2, Nazish Huq, MS2, Carl Spana, PhD2, Paul Kayne, PhD2. P5491 - Safety, Tolerability, and Efficacy of PL8177 in Adults With Active Ulcerative Colitis: Results From a Phase 2a, Randomized, Placebo-Controlled Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Dana J. Lukin, MD, PhD, FACG1, Robert Jordan, BS2, Nazish Huq, MS2, Carl Spana, PhD2, Paul Kayne, PhD2
1Jill Roberts Center for Inflammatory Bowel Disease, Weill Cornell Medicine, New York, NY; 2Palatin Technologies, Inc., Cranbury, NJ
Introduction: PL8177 is a synthetic, selective melanocortin receptor 1 (MC1R) agonist. The oral formulation contains polymers to inhibit degradation in the upper gastrointestinal (GI) tract and optimize lower GI release. In a rat model of ulcerative colitis (UC), PL8177 reduced colonic macroscopic damage, fecal occult blood, and colon weight and improved stool consistency. Here, we present clinical results from a phase 2a, double-blind, randomized, placebo-controlled, parallel group study of the safety, tolerability, and efficacy of PL8177 in adults with UC.
Methods: Participants aged 18-75 years with active, mild-to-moderate UC received PL8177 (20 mg) or placebo (randomized 3:1) once daily for 56 days. Endoscopic examination of the colon with biopsy was performed at baseline and at day 56. Blood and/or fecal samples were collected on days 1, 15, 29, and 56. Clinical response, clinical remission, and symptomatic remission were determined using the modified Mayo score (endoscopy, rectal bleeding, stool frequency) (Table). The primary endpoint was an endoscopic subscore of 0 or 1 (modified to exclude friability). Adverse events (AEs) were collected.
Results: A total of 64 participants were screened and 12 participants (PL8177, n=9; placebo, n=3) were randomized. Mean age was 47.1 years (range, 24-75) and 50.0% were female. A total of 12 AEs in 4 participants and 6 AEs in 1 participant were reported in PL8177 and placebo groups, respectively. All AEs were deemed not related or unlikely related to treatment. A clinical response was achieved in 7 of 9 participants (78%) in the PL8177 group compared with 1 of 3 (33%) in the placebo group (Figure). Clinical remission was achieved by 3 of 9 participants (33%) in the PL8177 group (vs none with placebo), while symptomatic remission was achieved by 5 of 9 (56%) with PL8177 (vs 1 of 3 [33%] with placebo). The planned primary endpoint was achieved by 3 of 9 participants (33%) with PL8177 and 1 of 3 (33%) with placebo.
Discussion: There is an unmet need for pharmacologic treatments that target novel, pathologic mechanisms of UC and/or inflammatory bowel disease. In this proof-of-principle phase 2a study, PL8177 provides broad efficacy at 8 weeks across key endpoints defined by the FDA (clinical remission and response) and also in symptomatic remission. The efficacy and excellent tolerability represent a highly differentiated profile and potential alternative to current immunosuppressive and steroid treatments.

Figure: Table. Definitions of Clinical Response, Clinical Remission, and Symptomatic Remission

Figure: Figure. Proportion of Participants to Achieve a Clinical Response, Clinical Remission, or Symptomatic Remission
Disclosures:
Dana Lukin: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Altrubio – Consultant. Boehringer Ingelheim – Consultant, Grant/Research Support. Bristol Myers Squibb – Consultant. Johnson & Johnson – Consultant, Grant/Research Support, Speakers Bureau. Palatin Technologies – Consultant. Pfizer – Consultant. Prime – Consultant. PSI – Consultant. Takeda – Consultant. Vedanta – Consultant.
Robert Jordan: Palatin Technologies, Inc. – Employee.
Nazish Huq: Palatin Technologies, Inc. – Employee.
Carl Spana: Palatin Technologies, Inc. – Employee.
Paul Kayne: Palatin Technologies, Inc. – Employee.
Dana J. Lukin, MD, PhD, FACG1, Robert Jordan, BS2, Nazish Huq, MS2, Carl Spana, PhD2, Paul Kayne, PhD2. P5491 - Safety, Tolerability, and Efficacy of PL8177 in Adults With Active Ulcerative Colitis: Results From a Phase 2a, Randomized, Placebo-Controlled Study, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

