Tuesday Poster Session
Category: Interventional Endoscopy
P5681 - Local Hemostatic Agents for Preventing Delayed Bleeding After Endoscopic Submucosal Dissection: A Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Khalid Ahmed, MD (he/him/his)
University of Minnesota
Minneapolis, MN
Presenting Author(s)
Khalid Ahmed, MD1, Fatima Elmustafa, MBBS2, Mouhand Mohamed, MBBS, MS3, Yasmin O. Ali, MBBS4, Abdelaziz Mohamed, MBBS5, Nicole Theis-Mahon, MLIS6, Mohamed Abdallah, MD7, Ahmed Dirweesh, MD6, Nabeel Azeem, MD1, Stuart K. Amateau, MD, PhD6
1University of Minnesota, Minneapolis, MN; 2Henry Ford Warren, Warren, MI; 3Mayo Clinic, Rochester, MN; 4Hennepin Healthcare, Minneapolis, MN; 5One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 6University of Minnesota Medical Center, Minneapolis, MN; 7Corewell Health, Royal Oak, MI
Introduction: Endoscopic submucosal dissection (ESD) is increasingly utilized for resection of gastrointestinal neoplasia but carries a risk of adverse events, particularly delayed bleeding. Various local hemostatic agents have been developed to reduce this risk; however, their efficacy across different gastrointestinal lesions has not been clearly established. We conducted a systematic review and meta-analysis of randomized controlled trials to evaluate the efficacy and safety of local hemostatic agents in preventing delayed post-ESD bleeding.
Methods: We conducted a comprehensive literature search of MEDLINE, EMBASE, Cochrane, Web of Science (Core Collection), and Scopus databases for randomized controlled trials (RCTs) evaluating the use of local hemostatic agents following ESD. Studies published in English from database inception through May 2025 were considered. Weighted pooled estimates with corresponding 95% confidence intervals (CI) were calculated using a random effects model. The main outcome was delayed bleeding.
Results: We identified 720 articles after duplicates were removed. A total of seven RCTs met the inclusion criteria and were included in the final analysis. Two studies were conducted in each of China, Japan, and South Korea, and one study was conducted in the United Kingdom. Across all studies, a total of 1,094 patients were included: 549 in the intervention group and 545 in the control group, with approximately 32.6% females (Table 1.). The overall rate of delayed bleeding from all included studies did not significantly differ between the intervention and control groups (risk ratio [RR] 1.04; 95% CI, 0.67–1.60; p=0.87, I2=0%) (Figure 1.). Three studies used gel-based hemostatic agents, and subgroup analysis of these studies demonstrated no significant difference in delayed bleeding risk between the two groups (RR 1.04; 95% CI, 0.52–2.08). When stratified by location, the pooled risk ratio for delated bleeding was 0.44 (95%CI, 0.05-3.77) for esophageal (2 studies), 0.96 (95% CI, 0.54-1.69) for gastric (5 studies), and 0.85 (95% CI, 0.10-7.32) for colorectal (2 studies). None of these subgroup differences reached statistical significance.
Discussion: Despite the clinical burden of delayed bleeding after ESD, current local hemostatic agents have not demonstrated consistent benefit. Further innovation and high-quality trials are needed to develop effective strategies to reduce bleeding-related morbidity and healthcare utilization.
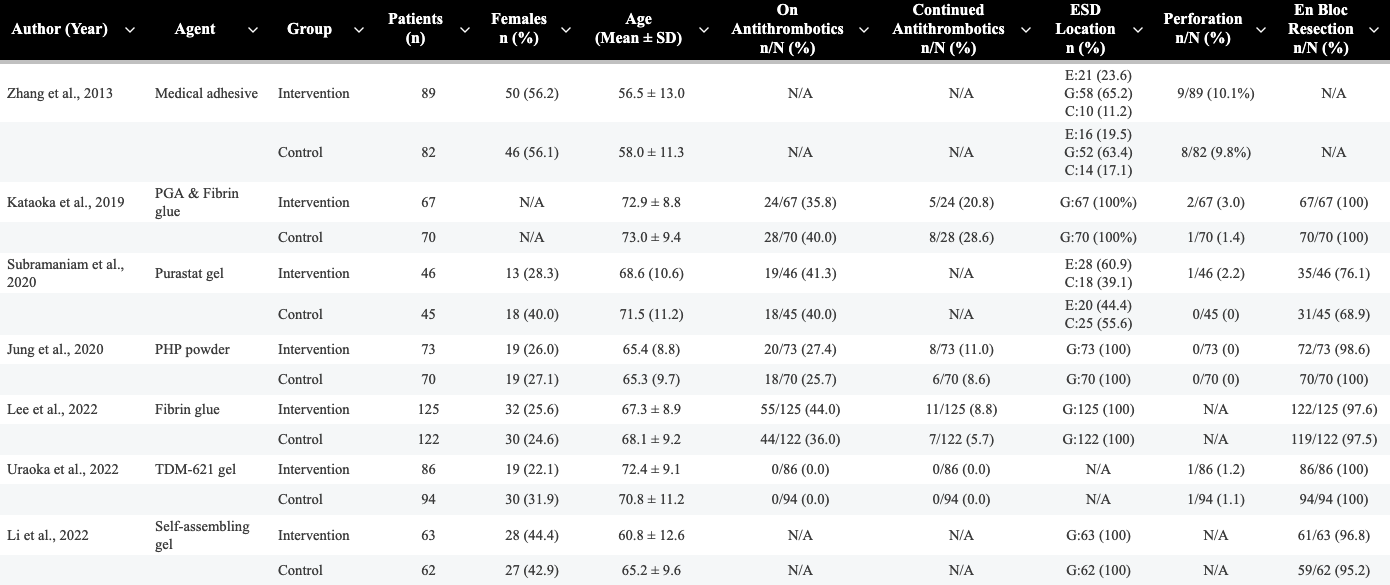
Figure: Table 1. Baseline characteristics of randomized controlled trials comparing local hemostatic agents to standard care following ESD.
ESD=Endoscopic Submucosal Dissection, G=Gastric, E=Esophageal, C=Colorectal.
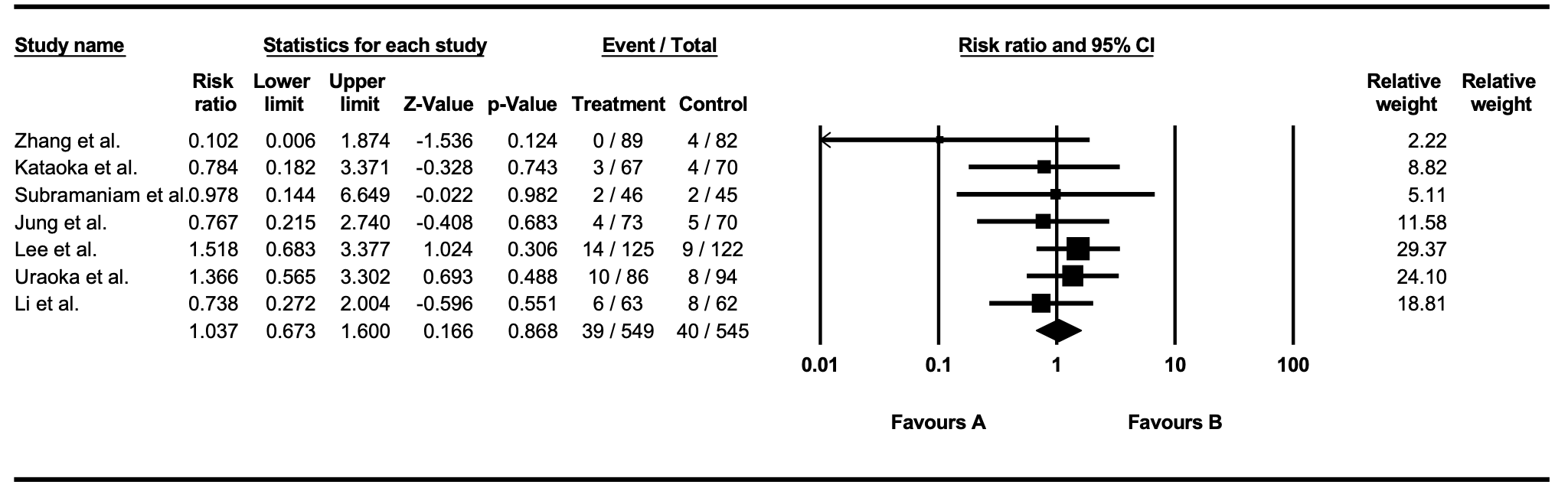
Figure:
Figure 1. Forest plot representing overall delayed bleeding
Disclosures:
Khalid Ahmed indicated no relevant financial relationships.
Fatima Elmustafa indicated no relevant financial relationships.
Mouhand Mohamed indicated no relevant financial relationships.
Yasmin Ali indicated no relevant financial relationships.
Abdelaziz Mohamed indicated no relevant financial relationships.
Nicole Theis-Mahon indicated no relevant financial relationships.
Mohamed Abdallah indicated no relevant financial relationships.
Ahmed Dirweesh indicated no relevant financial relationships.
Nabeel Azeem: Boston Scientific – Consultant.
Stuart Amateau: BSC – Consultant. Cook – Consultant. Endo-Therapeutics – Consultant. Merit – Consultant. Olympus – Advisor or Review Panel Member, Consultant. Provation – Advisory Committee/Board Member. Steris – Consultant.
Khalid Ahmed, MD1, Fatima Elmustafa, MBBS2, Mouhand Mohamed, MBBS, MS3, Yasmin O. Ali, MBBS4, Abdelaziz Mohamed, MBBS5, Nicole Theis-Mahon, MLIS6, Mohamed Abdallah, MD7, Ahmed Dirweesh, MD6, Nabeel Azeem, MD1, Stuart K. Amateau, MD, PhD6. P5681 - Local Hemostatic Agents for Preventing Delayed Bleeding After Endoscopic Submucosal Dissection: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Minnesota, Minneapolis, MN; 2Henry Ford Warren, Warren, MI; 3Mayo Clinic, Rochester, MN; 4Hennepin Healthcare, Minneapolis, MN; 5One Brooklyn Health-Interfaith Medical Center, Brooklyn, NY; 6University of Minnesota Medical Center, Minneapolis, MN; 7Corewell Health, Royal Oak, MI
Introduction: Endoscopic submucosal dissection (ESD) is increasingly utilized for resection of gastrointestinal neoplasia but carries a risk of adverse events, particularly delayed bleeding. Various local hemostatic agents have been developed to reduce this risk; however, their efficacy across different gastrointestinal lesions has not been clearly established. We conducted a systematic review and meta-analysis of randomized controlled trials to evaluate the efficacy and safety of local hemostatic agents in preventing delayed post-ESD bleeding.
Methods: We conducted a comprehensive literature search of MEDLINE, EMBASE, Cochrane, Web of Science (Core Collection), and Scopus databases for randomized controlled trials (RCTs) evaluating the use of local hemostatic agents following ESD. Studies published in English from database inception through May 2025 were considered. Weighted pooled estimates with corresponding 95% confidence intervals (CI) were calculated using a random effects model. The main outcome was delayed bleeding.
Results: We identified 720 articles after duplicates were removed. A total of seven RCTs met the inclusion criteria and were included in the final analysis. Two studies were conducted in each of China, Japan, and South Korea, and one study was conducted in the United Kingdom. Across all studies, a total of 1,094 patients were included: 549 in the intervention group and 545 in the control group, with approximately 32.6% females (Table 1.). The overall rate of delayed bleeding from all included studies did not significantly differ between the intervention and control groups (risk ratio [RR] 1.04; 95% CI, 0.67–1.60; p=0.87, I2=0%) (Figure 1.). Three studies used gel-based hemostatic agents, and subgroup analysis of these studies demonstrated no significant difference in delayed bleeding risk between the two groups (RR 1.04; 95% CI, 0.52–2.08). When stratified by location, the pooled risk ratio for delated bleeding was 0.44 (95%CI, 0.05-3.77) for esophageal (2 studies), 0.96 (95% CI, 0.54-1.69) for gastric (5 studies), and 0.85 (95% CI, 0.10-7.32) for colorectal (2 studies). None of these subgroup differences reached statistical significance.
Discussion: Despite the clinical burden of delayed bleeding after ESD, current local hemostatic agents have not demonstrated consistent benefit. Further innovation and high-quality trials are needed to develop effective strategies to reduce bleeding-related morbidity and healthcare utilization.

Figure: Table 1. Baseline characteristics of randomized controlled trials comparing local hemostatic agents to standard care following ESD.
ESD=Endoscopic Submucosal Dissection, G=Gastric, E=Esophageal, C=Colorectal.

Figure:
Figure 1. Forest plot representing overall delayed bleeding
Disclosures:
Khalid Ahmed indicated no relevant financial relationships.
Fatima Elmustafa indicated no relevant financial relationships.
Mouhand Mohamed indicated no relevant financial relationships.
Yasmin Ali indicated no relevant financial relationships.
Abdelaziz Mohamed indicated no relevant financial relationships.
Nicole Theis-Mahon indicated no relevant financial relationships.
Mohamed Abdallah indicated no relevant financial relationships.
Ahmed Dirweesh indicated no relevant financial relationships.
Nabeel Azeem: Boston Scientific – Consultant.
Stuart Amateau: BSC – Consultant. Cook – Consultant. Endo-Therapeutics – Consultant. Merit – Consultant. Olympus – Advisor or Review Panel Member, Consultant. Provation – Advisory Committee/Board Member. Steris – Consultant.
Khalid Ahmed, MD1, Fatima Elmustafa, MBBS2, Mouhand Mohamed, MBBS, MS3, Yasmin O. Ali, MBBS4, Abdelaziz Mohamed, MBBS5, Nicole Theis-Mahon, MLIS6, Mohamed Abdallah, MD7, Ahmed Dirweesh, MD6, Nabeel Azeem, MD1, Stuart K. Amateau, MD, PhD6. P5681 - Local Hemostatic Agents for Preventing Delayed Bleeding After Endoscopic Submucosal Dissection: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
