Tuesday Poster Session
Category: Liver
P5809 - Comparative Effectiveness of Immunotherapy vs Sorafenib in Advanced Hepatocellular Carcinoma: A Multi-Regimen Survival and Response Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Prince Shah-Riar, MD (he/him/his)
DHR Health, Edinburg, Tx
Edinburg, TX
Presenting Author(s)
Prince Shah-Riar, MD1, Abdul Baseet Arham, MBBS2, Sumaiya Ahmed, MBBS3, Sumaiya Monjur, MBBS4, Farjana Masud, MBBS5, Asif Zamir, MD, FACG6
1DHR Health, Edinburg, Tx, McAllen, TX; 2Weill Cornell Medical College, New York, NY; 3North South University, Tallahassee, FL; 4Dhaka Medical College and Hospital, Spokane, WA; 5Shaheed Ziaur Rahman Medical College, Bangladesh, Rome, NY; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide, especially when diagnosed at advanced stages with limited curative options. While Sorafenib has served as the standard systemic therapy, emerging immunotherapy regimens show potential for improved outcomes. However, comprehensive statistical comparisons among leading immunotherapy regimens remain underexplored.Although large randomized controlled trials (RCTs) like IMbrave150 and CheckMate 459 suggest improved outcomes with immunotherapy, consolidated statistical comparisons across multiple regimens—including recent combinations—are lacking in the literature. Specifically, meta-analytical evaluations of their effects on overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) relative to Sorafenib are limited. This study aimed to assess and compare the clinical effectiveness of immunotherapy regimens versus Sorafenib in the treatment of unresectable HCC, focusing on OS, PFS, and ORR.
Methods: A comparative statistical analysis using single-factor ANOVA (α = 0.05) was performed on efficacy data extracted from published phase III RCTs. Treatment regimens analyzed included:
The primary endpoints were OS, PFS, and ORR.
Results:
Discussion: While OS and PFS outcomes did not significantly differ across treatment regimens, immunotherapy combinations—particularly Lenvatinib + Pembrolizumab—exhibited a statistically significant and clinically meaningful improvement in ORR. These findings support the use of immunotherapy in patients for whom tumor burden reduction is a key therapeutic goal. Further investigation is warranted to establish durable survival benefits and to refine patient selection for immunotherapy-based strategies in advanced HCC.
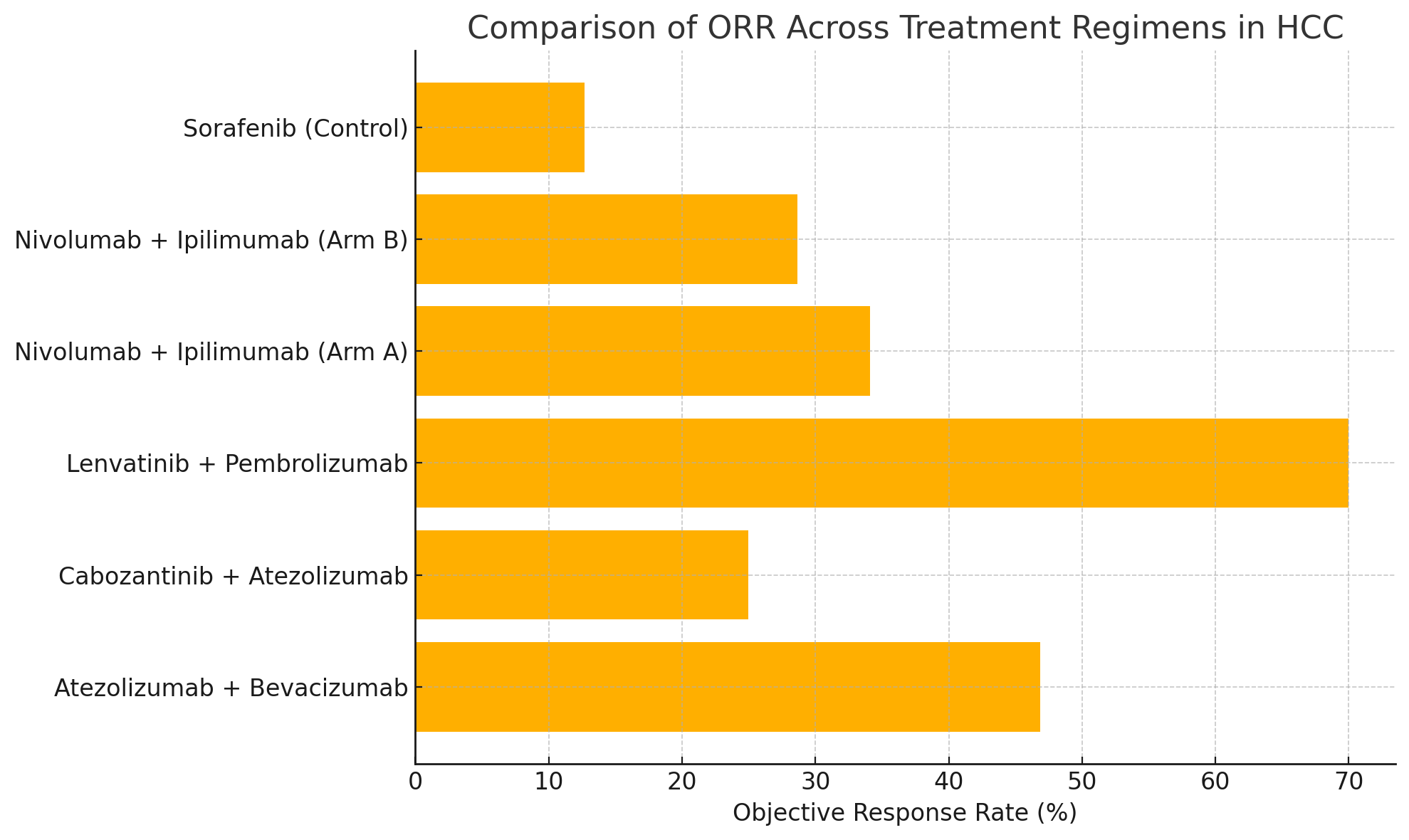
Figure: Figure 1. Comparison of Objective Response Rate (ORR) across HCC treatment regimens.
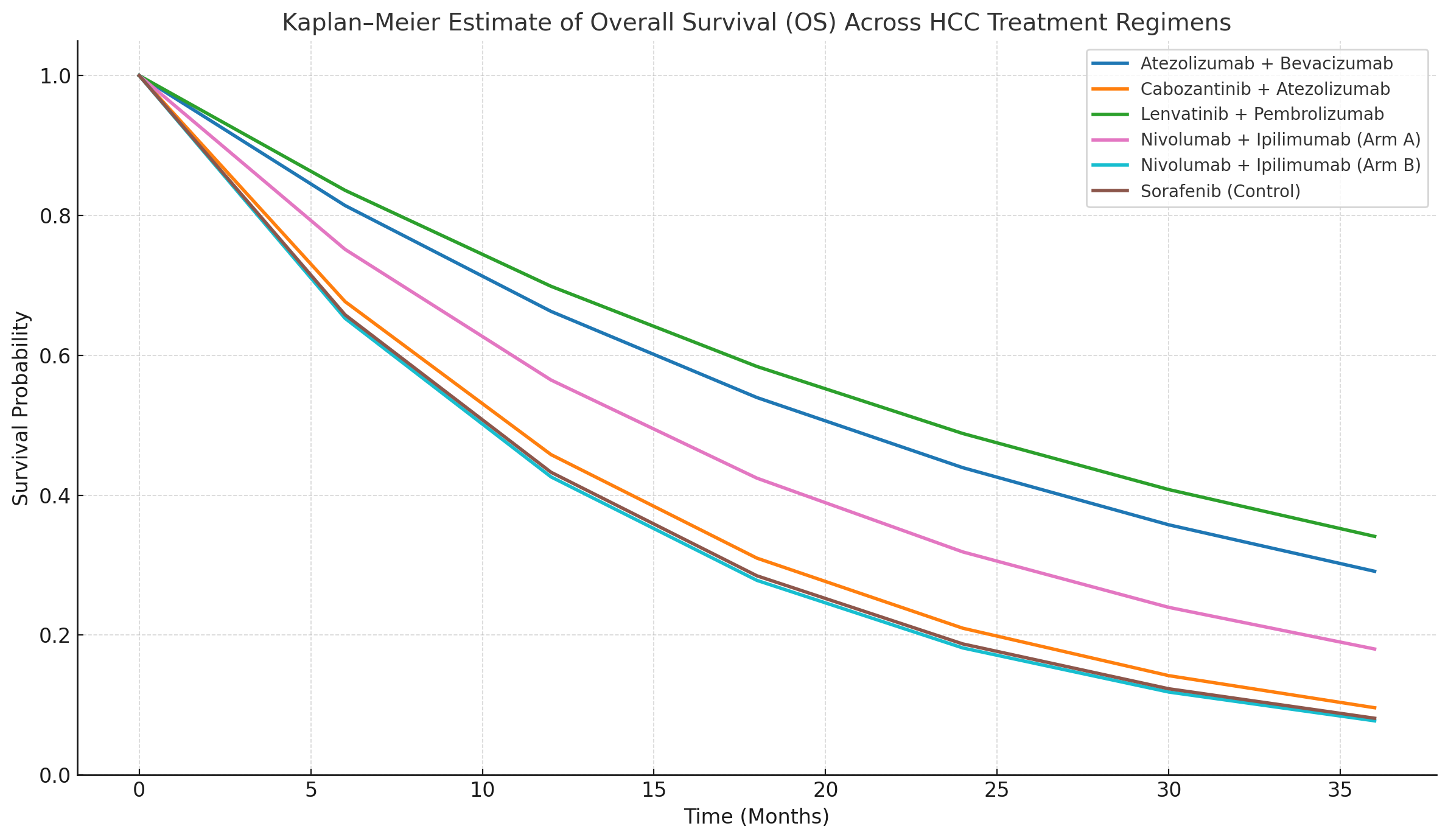
Figure: Figure 2. Kaplan–Meier estimates of Overall Survival (OS) by treatment group.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Abdul Baseet Arham indicated no relevant financial relationships.
Sumaiya Ahmed indicated no relevant financial relationships.
Sumaiya Monjur indicated no relevant financial relationships.
Farjana Masud indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Abdul Baseet Arham, MBBS2, Sumaiya Ahmed, MBBS3, Sumaiya Monjur, MBBS4, Farjana Masud, MBBS5, Asif Zamir, MD, FACG6. P5809 - Comparative Effectiveness of Immunotherapy vs Sorafenib in Advanced Hepatocellular Carcinoma: A Multi-Regimen Survival and Response Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1DHR Health, Edinburg, Tx, McAllen, TX; 2Weill Cornell Medical College, New York, NY; 3North South University, Tallahassee, FL; 4Dhaka Medical College and Hospital, Spokane, WA; 5Shaheed Ziaur Rahman Medical College, Bangladesh, Rome, NY; 6DHR Health Gastroenterology, Edinburg, TX
Introduction: Hepatocellular carcinoma (HCC) remains a leading cause of cancer-related mortality worldwide, especially when diagnosed at advanced stages with limited curative options. While Sorafenib has served as the standard systemic therapy, emerging immunotherapy regimens show potential for improved outcomes. However, comprehensive statistical comparisons among leading immunotherapy regimens remain underexplored.Although large randomized controlled trials (RCTs) like IMbrave150 and CheckMate 459 suggest improved outcomes with immunotherapy, consolidated statistical comparisons across multiple regimens—including recent combinations—are lacking in the literature. Specifically, meta-analytical evaluations of their effects on overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) relative to Sorafenib are limited. This study aimed to assess and compare the clinical effectiveness of immunotherapy regimens versus Sorafenib in the treatment of unresectable HCC, focusing on OS, PFS, and ORR.
Methods: A comparative statistical analysis using single-factor ANOVA (α = 0.05) was performed on efficacy data extracted from published phase III RCTs. Treatment regimens analyzed included:
- Nivolumab + Ipilimumab
- Atezolizumab + Bevacizumab
- Cabozantinib + Atezolizumab
- Lenvatinib + Pembrolizumab
- Sorafenib (control group)
The primary endpoints were OS, PFS, and ORR.
Results:
- Overall Survival (OS): No statistically significant difference across regimens (p = 0.6896).
- Progression-Free Survival (PFS): A trend favoring immunotherapy combinations was observed but did not reach statistical significance (p = 0.0613).
- Objective Response Rate (ORR): Statistically significant differences were observed (p < 0.0001). The Lenvatinib + Pembrolizumab group demonstrated the highest ORR at 70%, compared to 12.67% with Sorafenib (see Figure 1). Kaplan–Meier estimates for OS are presented in Figure 2.
Discussion: While OS and PFS outcomes did not significantly differ across treatment regimens, immunotherapy combinations—particularly Lenvatinib + Pembrolizumab—exhibited a statistically significant and clinically meaningful improvement in ORR. These findings support the use of immunotherapy in patients for whom tumor burden reduction is a key therapeutic goal. Further investigation is warranted to establish durable survival benefits and to refine patient selection for immunotherapy-based strategies in advanced HCC.

Figure: Figure 1. Comparison of Objective Response Rate (ORR) across HCC treatment regimens.

Figure: Figure 2. Kaplan–Meier estimates of Overall Survival (OS) by treatment group.
Disclosures:
Prince Shah-Riar indicated no relevant financial relationships.
Abdul Baseet Arham indicated no relevant financial relationships.
Sumaiya Ahmed indicated no relevant financial relationships.
Sumaiya Monjur indicated no relevant financial relationships.
Farjana Masud indicated no relevant financial relationships.
Asif Zamir indicated no relevant financial relationships.
Prince Shah-Riar, MD1, Abdul Baseet Arham, MBBS2, Sumaiya Ahmed, MBBS3, Sumaiya Monjur, MBBS4, Farjana Masud, MBBS5, Asif Zamir, MD, FACG6. P5809 - Comparative Effectiveness of Immunotherapy vs Sorafenib in Advanced Hepatocellular Carcinoma: A Multi-Regimen Survival and Response Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
