Tuesday Poster Session
Category: Liver
P5889 - Efficacy and Safety of GLP-1 Receptor Agonists in Metabolic-Associated Steatohepatitis With Significant Fibrosis or Cirrhosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Sudheer Dhoop, MD
University of Toledo College Medicine and Life Sciences
Toledo, Ohio
Presenting Author(s)
Sudheer Dhoop, MD1, Bisher Sawaf, MD2, Maram Albandak, MD3, Alexander Arch, BS4, Priya Vadehra, BS5, Wasef Sayeh, MD4, Mona Hassan, MD6
1University of Toledo College Medicine and Life Sciences, Toledo, OH; 2University of Toledo Medical Center, Toledo, OH; 3The University of Toledo, Toledo, OH; 4University of Toledo College of Medicine and Life Sciences, Toledo, OH; 5Wayne State University, Livonia, MI; 6Division of Gastroenterology & Hepatology, Toledo, OH
Introduction: Glucagon-Like Peptide Receptor Agonists (GLP-1 RAs) are approved for the FDA for treating Type 2 Diabetes and Obesity, now popular for their simultaneous cardiovascular benefit. Resmetirom, however, is the only approved treatment for Metabolic Associated Steatohepatitis (MASH) in F2-F3 stages of fibrosis. Our study assesses the efficacy and safety of GLP-1 RAs for a similar range of patients.
Methods: A comprehensive search was constructed in Embase on May 1, 2025 and translated to other major health databases. Two authors screened records to identify those where all patients were classified as having metabolic-associated steatohepatitis with results for F2 or higher stages of fibrosis. Reviews, observational studies, and case reports were excluded. Meta-analysis was conducted in RevMan v5.4.1 with pooling of dichotomous outcomes using the random effects model, generating odds ratios as effect sizes. The primary outcome was the resolution of steatohepatitis without worsening of fibrosis. Secondary outcomes were improvement of fibrosis by at least one grade without evidence of steatohepatitis and a composite of gastrointestinal events including nausea, vomiting, dyspepsia, and abdominal pain. Subgrouping was done for patients with and without cirrhosis.
Results: Our review included 4 studies involving 1,177 patients with 6% having compensated cirrhosis (Table 1). Resolution of steatohepatitis occurred in the non-cirrhotic group (OR= 5.40, CI: 2.58-11.29, p< 0.01, I2=67%) but not in the cirrhosis study (OR=1.96, CI: 0.62-6.23, p=0.25) (see Figure 1C). Improvement of fibrosis occurred in the non-cirrhotic group (OR=1.99, CI: 1.49-2.65, p< 0.01, I2=0%), but not the cirrhosis study (OR=1.48, CI: 0.48-4.78, p=0.51) (Figure 1B). Adverse events were increased in the non-cirrhotic group (OR=4.92, CI: 2.60-9.30, p< 0.01, I2=62%) and the cirrhosis study (OR=4.36, CI: 1.53-12.39, p< 0.01) to a similar degree χ² =0.04 (p=0.85) (see Figure 1C).
Discussion: Our study reveals that GLP-1 RAs are efficacious in resolution of steatohepatitis and improvement of fibrosis in the F2-F3 stages of fibrosis, but not cirrhosis highlighting the importance of identifying MASLD early prior to the development of cirrhosis. The adverse event profile remains similar in both populations, however, demonstrating GLP-1 RAs are similarly tolerated in compensated cirrhosis and can be continued for other indications. Further trials on GLP-1 RAs are needed to confirm their lack of efficacy in treating MASH in cirrhosis.
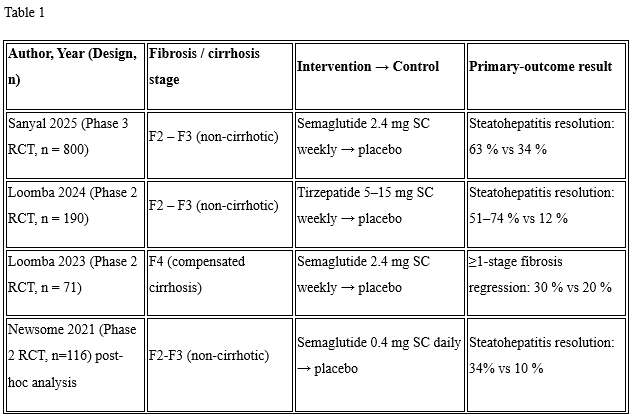
Figure: Table 1. Studies Assessing GLP-1 RAs Efficacy in Metabolic Associated Steatohepatitis including Cirrhosis populations
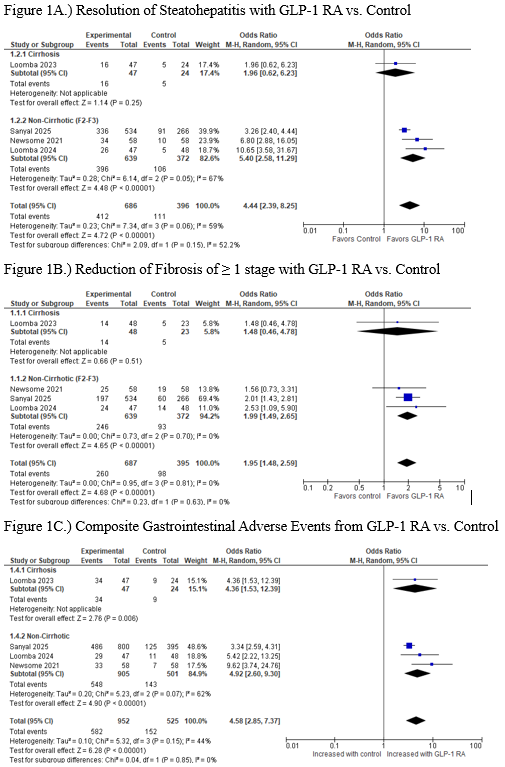
Figure: Figure 1. A) Resolution of Steatohepatitis with GLP-1 RA vs. Control. B.) Improvement in at least one stage of fibrosis with GLP-1 RA vs. Control C.) Composite of Gastrointestinal Adverse Events from GLP-1 RA vs. Control.
Disclosures:
Sudheer Dhoop indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Maram Albandak indicated no relevant financial relationships.
Alexander Arch indicated no relevant financial relationships.
Priya Vadehra indicated no relevant financial relationships.
Wasef Sayeh indicated no relevant financial relationships.
Mona Hassan indicated no relevant financial relationships.
Sudheer Dhoop, MD1, Bisher Sawaf, MD2, Maram Albandak, MD3, Alexander Arch, BS4, Priya Vadehra, BS5, Wasef Sayeh, MD4, Mona Hassan, MD6. P5889 - Efficacy and Safety of GLP-1 Receptor Agonists in Metabolic-Associated Steatohepatitis With Significant Fibrosis or Cirrhosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Toledo College Medicine and Life Sciences, Toledo, OH; 2University of Toledo Medical Center, Toledo, OH; 3The University of Toledo, Toledo, OH; 4University of Toledo College of Medicine and Life Sciences, Toledo, OH; 5Wayne State University, Livonia, MI; 6Division of Gastroenterology & Hepatology, Toledo, OH
Introduction: Glucagon-Like Peptide Receptor Agonists (GLP-1 RAs) are approved for the FDA for treating Type 2 Diabetes and Obesity, now popular for their simultaneous cardiovascular benefit. Resmetirom, however, is the only approved treatment for Metabolic Associated Steatohepatitis (MASH) in F2-F3 stages of fibrosis. Our study assesses the efficacy and safety of GLP-1 RAs for a similar range of patients.
Methods: A comprehensive search was constructed in Embase on May 1, 2025 and translated to other major health databases. Two authors screened records to identify those where all patients were classified as having metabolic-associated steatohepatitis with results for F2 or higher stages of fibrosis. Reviews, observational studies, and case reports were excluded. Meta-analysis was conducted in RevMan v5.4.1 with pooling of dichotomous outcomes using the random effects model, generating odds ratios as effect sizes. The primary outcome was the resolution of steatohepatitis without worsening of fibrosis. Secondary outcomes were improvement of fibrosis by at least one grade without evidence of steatohepatitis and a composite of gastrointestinal events including nausea, vomiting, dyspepsia, and abdominal pain. Subgrouping was done for patients with and without cirrhosis.
Results: Our review included 4 studies involving 1,177 patients with 6% having compensated cirrhosis (Table 1). Resolution of steatohepatitis occurred in the non-cirrhotic group (OR= 5.40, CI: 2.58-11.29, p< 0.01, I2=67%) but not in the cirrhosis study (OR=1.96, CI: 0.62-6.23, p=0.25) (see Figure 1C). Improvement of fibrosis occurred in the non-cirrhotic group (OR=1.99, CI: 1.49-2.65, p< 0.01, I2=0%), but not the cirrhosis study (OR=1.48, CI: 0.48-4.78, p=0.51) (Figure 1B). Adverse events were increased in the non-cirrhotic group (OR=4.92, CI: 2.60-9.30, p< 0.01, I2=62%) and the cirrhosis study (OR=4.36, CI: 1.53-12.39, p< 0.01) to a similar degree χ² =0.04 (p=0.85) (see Figure 1C).
Discussion: Our study reveals that GLP-1 RAs are efficacious in resolution of steatohepatitis and improvement of fibrosis in the F2-F3 stages of fibrosis, but not cirrhosis highlighting the importance of identifying MASLD early prior to the development of cirrhosis. The adverse event profile remains similar in both populations, however, demonstrating GLP-1 RAs are similarly tolerated in compensated cirrhosis and can be continued for other indications. Further trials on GLP-1 RAs are needed to confirm their lack of efficacy in treating MASH in cirrhosis.

Figure: Table 1. Studies Assessing GLP-1 RAs Efficacy in Metabolic Associated Steatohepatitis including Cirrhosis populations

Figure: Figure 1. A) Resolution of Steatohepatitis with GLP-1 RA vs. Control. B.) Improvement in at least one stage of fibrosis with GLP-1 RA vs. Control C.) Composite of Gastrointestinal Adverse Events from GLP-1 RA vs. Control.
Disclosures:
Sudheer Dhoop indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Maram Albandak indicated no relevant financial relationships.
Alexander Arch indicated no relevant financial relationships.
Priya Vadehra indicated no relevant financial relationships.
Wasef Sayeh indicated no relevant financial relationships.
Mona Hassan indicated no relevant financial relationships.
Sudheer Dhoop, MD1, Bisher Sawaf, MD2, Maram Albandak, MD3, Alexander Arch, BS4, Priya Vadehra, BS5, Wasef Sayeh, MD4, Mona Hassan, MD6. P5889 - Efficacy and Safety of GLP-1 Receptor Agonists in Metabolic-Associated Steatohepatitis With Significant Fibrosis or Cirrhosis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
