Tuesday Poster Session
Category: Liver
P5882 - Efficacy and Safety of Efruxifermin in Patients With MASH: A Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Islam Rajab, MD
St. Joseph's University Medical Center
Paterson, NJ
Presenting Author(s)
Islam Rajab, MD1, Mohamed S. Elgendy, MBBCh2, Ahmed Emara, 3, Abdalhakim Shubietah, MD4, Mohamed Saad Rakab, 5, Ameer Awashra, 6, Ahmed Abuhasna, DO4, Abdallah Hussein, MD7, Ahmed Salem, MD8, Dhruv Patel, 1, Furqan A Bhullar, 1, Patrick Tempera, DO1, Walid Baddoura, MD1, Yana Cavanagh, MD1
1St. Joseph's University Medical Center, Paterson, NJ; 2Tanta University, Tanta, Al Gharbiyah, Egypt; 3Faculty of Medicine, Al-Azhar University, Cairo, Egypt, Cairo, Al Qahirah, Egypt; 4Advocate Illinois Masonic Medical Center, Chicago, IL; 5Faculty of Medicine, Mansoura University, Mansoura, Egypt, Mansoura, Ad Daqahliyah, Egypt; 6An-Najah National University Faculty of Medicine and Health Sciences, Nablus, Palestinian Territories; 7Virtua Our Lady of Lourdes Hospital, Camden, NJ; 8Maimonides Medical Center, Brooklyn, NY
Introduction: Metabolic dysfunction-associated steatohepatitis (MASH) is a progressive liver disease marked by steatosis, inflammation, and fibrosis, with few approved treatment options. Efruxifermin, an FGF21 analog, has shown potential in improving both metabolic and histological features of MASH. This analysis evaluates its efficacy across key histological outcomes compared to placebo.
Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted by searching PubMed, Scopus, Web of Science, and Cochrane CENTRAL through May 28, 2025. Eligible studies included adult patients with MASH and compared efruxifermin with placebo. Meta-analyses were performed using risk ratios (RRs) with 95% confidence intervals for dichotomous outcomes. All statistical analyses were performed using Stata version 19
Results: Four randomized controlled trials comprising 419 patients were included, with 284 patients receiving efruxifermin and 135 receiving placebo. Efruxifermin significantly increased the likelihood of MASH resolution without worsening (RR = 2.42; 95% CI: 1.48–3.94; p < 0.001), ≥1-stage fibrosis improvement without MASH worsening (RR = 1.83; 95% CI: 1.17–2.84; p = 0.01). It also led to significant improvements in MASH resolution and improvement (RR = 3.77; 95% CI: 1.10–12.87; p = 0.03). However, ≥2-stage fibrosis improvement without MASH worsening was not statistically significant Figure1 . Efruxifermin was associated with a higher risk of drug-related adverse and discontinuations due to adverse events, while no significant differences were observed in serious adverse events, deaths, or overall treatment-emergent adverse events Figure 2.
Discussion: Efruxifermin was associated with improvements in fibrosis, MASH resolution compared to placebo. Additional RCTs are warranted to validate these effects and assess long-term efficacy and safety.
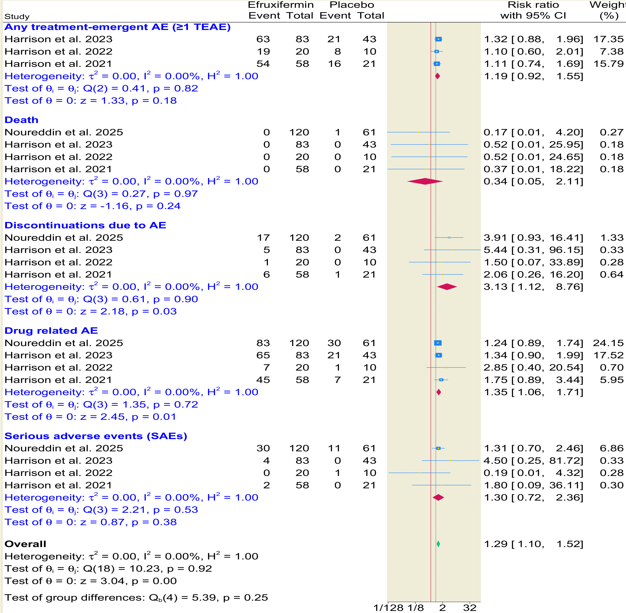
Figure: Figure1.Forest plots showing histological and biomarker outcomes comparing efruxifermin to placebo in patients with MASH.
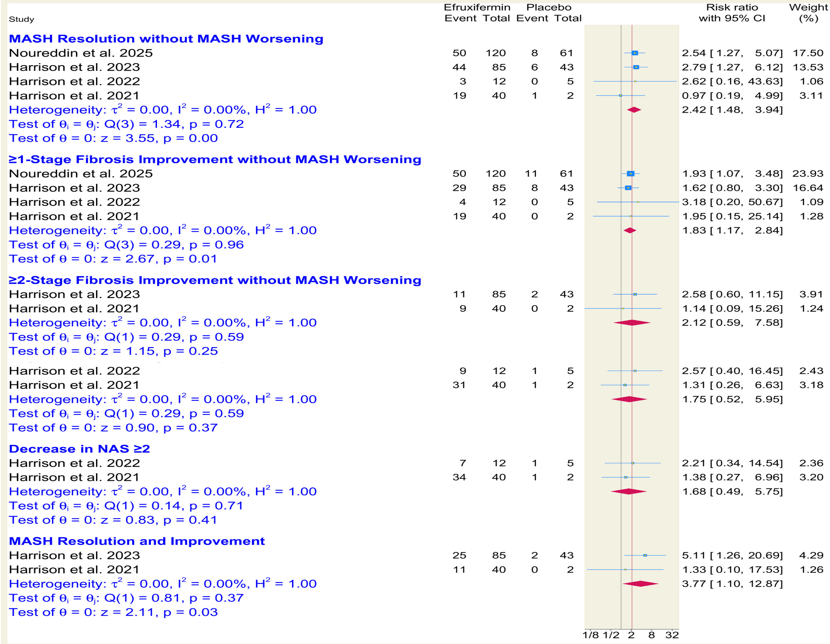
Figure: Figure 2.Forest plots showing safety outcomes comparing efruxifermin to placebo.
Forest plots showing safety outcomes comparing efruxifermin to placebo.
Disclosures:
Islam Rajab indicated no relevant financial relationships.
Mohamed S. Elgendy indicated no relevant financial relationships.
Ahmed Emara indicated no relevant financial relationships.
Abdalhakim Shubietah indicated no relevant financial relationships.
Mohamed Saad Rakab indicated no relevant financial relationships.
Ameer Awashra indicated no relevant financial relationships.
Ahmed Abuhasna indicated no relevant financial relationships.
Abdallah Hussein indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Dhruv Patel indicated no relevant financial relationships.
Furqan A Bhullar indicated no relevant financial relationships.
Patrick Tempera indicated no relevant financial relationships.
Walid Baddoura indicated no relevant financial relationships.
Yana Cavanagh indicated no relevant financial relationships.
Islam Rajab, MD1, Mohamed S. Elgendy, MBBCh2, Ahmed Emara, 3, Abdalhakim Shubietah, MD4, Mohamed Saad Rakab, 5, Ameer Awashra, 6, Ahmed Abuhasna, DO4, Abdallah Hussein, MD7, Ahmed Salem, MD8, Dhruv Patel, 1, Furqan A Bhullar, 1, Patrick Tempera, DO1, Walid Baddoura, MD1, Yana Cavanagh, MD1. P5882 - Efficacy and Safety of Efruxifermin in Patients With MASH: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1St. Joseph's University Medical Center, Paterson, NJ; 2Tanta University, Tanta, Al Gharbiyah, Egypt; 3Faculty of Medicine, Al-Azhar University, Cairo, Egypt, Cairo, Al Qahirah, Egypt; 4Advocate Illinois Masonic Medical Center, Chicago, IL; 5Faculty of Medicine, Mansoura University, Mansoura, Egypt, Mansoura, Ad Daqahliyah, Egypt; 6An-Najah National University Faculty of Medicine and Health Sciences, Nablus, Palestinian Territories; 7Virtua Our Lady of Lourdes Hospital, Camden, NJ; 8Maimonides Medical Center, Brooklyn, NY
Introduction: Metabolic dysfunction-associated steatohepatitis (MASH) is a progressive liver disease marked by steatosis, inflammation, and fibrosis, with few approved treatment options. Efruxifermin, an FGF21 analog, has shown potential in improving both metabolic and histological features of MASH. This analysis evaluates its efficacy across key histological outcomes compared to placebo.
Methods: A systematic review and meta-analysis of randomized controlled trials (RCTs) was conducted by searching PubMed, Scopus, Web of Science, and Cochrane CENTRAL through May 28, 2025. Eligible studies included adult patients with MASH and compared efruxifermin with placebo. Meta-analyses were performed using risk ratios (RRs) with 95% confidence intervals for dichotomous outcomes. All statistical analyses were performed using Stata version 19
Results: Four randomized controlled trials comprising 419 patients were included, with 284 patients receiving efruxifermin and 135 receiving placebo. Efruxifermin significantly increased the likelihood of MASH resolution without worsening (RR = 2.42; 95% CI: 1.48–3.94; p < 0.001), ≥1-stage fibrosis improvement without MASH worsening (RR = 1.83; 95% CI: 1.17–2.84; p = 0.01). It also led to significant improvements in MASH resolution and improvement (RR = 3.77; 95% CI: 1.10–12.87; p = 0.03). However, ≥2-stage fibrosis improvement without MASH worsening was not statistically significant Figure1 . Efruxifermin was associated with a higher risk of drug-related adverse and discontinuations due to adverse events, while no significant differences were observed in serious adverse events, deaths, or overall treatment-emergent adverse events Figure 2.
Discussion: Efruxifermin was associated with improvements in fibrosis, MASH resolution compared to placebo. Additional RCTs are warranted to validate these effects and assess long-term efficacy and safety.

Figure: Figure1.Forest plots showing histological and biomarker outcomes comparing efruxifermin to placebo in patients with MASH.

Figure: Figure 2.Forest plots showing safety outcomes comparing efruxifermin to placebo.
Forest plots showing safety outcomes comparing efruxifermin to placebo.
Disclosures:
Islam Rajab indicated no relevant financial relationships.
Mohamed S. Elgendy indicated no relevant financial relationships.
Ahmed Emara indicated no relevant financial relationships.
Abdalhakim Shubietah indicated no relevant financial relationships.
Mohamed Saad Rakab indicated no relevant financial relationships.
Ameer Awashra indicated no relevant financial relationships.
Ahmed Abuhasna indicated no relevant financial relationships.
Abdallah Hussein indicated no relevant financial relationships.
Ahmed Salem indicated no relevant financial relationships.
Dhruv Patel indicated no relevant financial relationships.
Furqan A Bhullar indicated no relevant financial relationships.
Patrick Tempera indicated no relevant financial relationships.
Walid Baddoura indicated no relevant financial relationships.
Yana Cavanagh indicated no relevant financial relationships.
Islam Rajab, MD1, Mohamed S. Elgendy, MBBCh2, Ahmed Emara, 3, Abdalhakim Shubietah, MD4, Mohamed Saad Rakab, 5, Ameer Awashra, 6, Ahmed Abuhasna, DO4, Abdallah Hussein, MD7, Ahmed Salem, MD8, Dhruv Patel, 1, Furqan A Bhullar, 1, Patrick Tempera, DO1, Walid Baddoura, MD1, Yana Cavanagh, MD1. P5882 - Efficacy and Safety of Efruxifermin in Patients With MASH: A Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
