Tuesday Poster Session
Category: Liver
P5938 - Real-World Safety and Monitoring Practices in Patients Receiving Resmetirom (Rezdiffra) for MASH: A Single Center Retrospective Review
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Dauris De Jesus Rosario Lora, MD
Rush University Medical Center
Chicago, IL
Presenting Author(s)
Dauris De Jesus. Rosario Lora, MD, Alivia Blumenstein, MD, Zoe Post, MD, MSc, Sujit Janardhan, MD, PhD, Nancy Reau, MD, FACG
Rush University Medical Center, Chicago, IL
Introduction: Resmetirom (Rezdiffra) is a selective thyroid hormone receptor-β agonist recently approved for the treatment of MASH (Metabolic dysfunction-associated steatohepatitis) with F2/3 fibrosis. While effective in clinical trials, real-world data on safety and adherence to monitoring guidelines remain limited given the medication’s recent market availability.
Methods: We conducted a retrospective analysis of patients prescribed Resmetirom at a single academic center. Data collection included demographics, treatment duration, adverse events, and adherence to laboratory monitoring guidelines, focusing on liver enzymes LFTs), fibrosis assessment and thyroid function. Patients without accessible laboratory data were excluded. A paired t-test was used for statistical analysis of NALFD Fibrosis Scores.
Results: The final sample included 34 patients. Of the initial cohort of 56 patients, 9 patients (16.1%) could not initiate therapy due to insurance denial, while the rest had either not started the drug, or had no laboratory data available. Patient characteristics are summarized in Table 1. The mean duration of therapy was 236.6 days. NAFLD fibrosis scores showed no significant change from baseline to 3–6 months (–1.17 vs. –1.21, p > 0.05), likely due to the short interval. Adverse events (Figure 1) were reported in 15 patients (44.1%), with gastrointestinal symptoms being the most common (10/15, 66.7%). Diarrhea accounted for the majority of GI complaints (6/10, 60%). There were three documented drug discontinuations, all due to diarrhea.
Monitoring practices varied. While 70% of patients had both baseline and follow-up LFTs at 3–6 months, only 38% had baseline thyroid function tests, and 14% had follow-up thyroid labs. One patient with preexisting hyperthyroidism required a levothyroxine dose adjustment after initiating therapy.
Discussion: In this real-world cohort, Resmetirom was generally well tolerated but associated with a notable rate of gastrointestinal side effects, particularly diarrhea. Monitoring practices frequently fell short of AASLD-recommended guidelines, highlighting the need for standardized protocols to ensure safety and optimize outcomes. Larger longitudinal studies are needed to better assess long-term safety and efficacy in routine clinical settings.
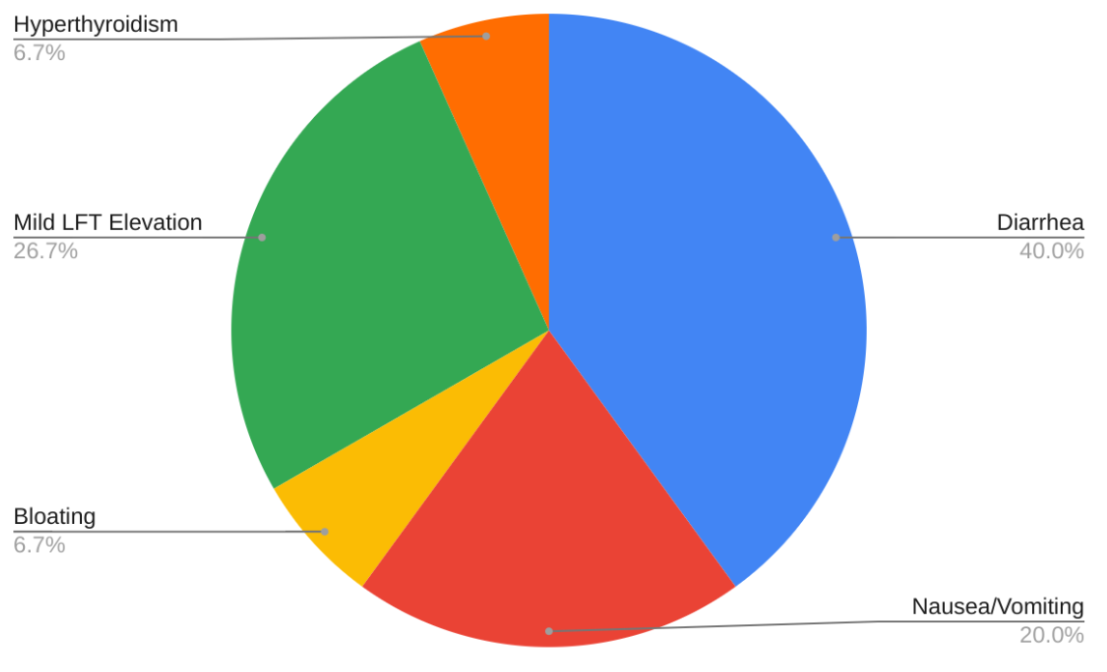
Figure: Figure 1. Documented Adverse Events after Resmetirom Start
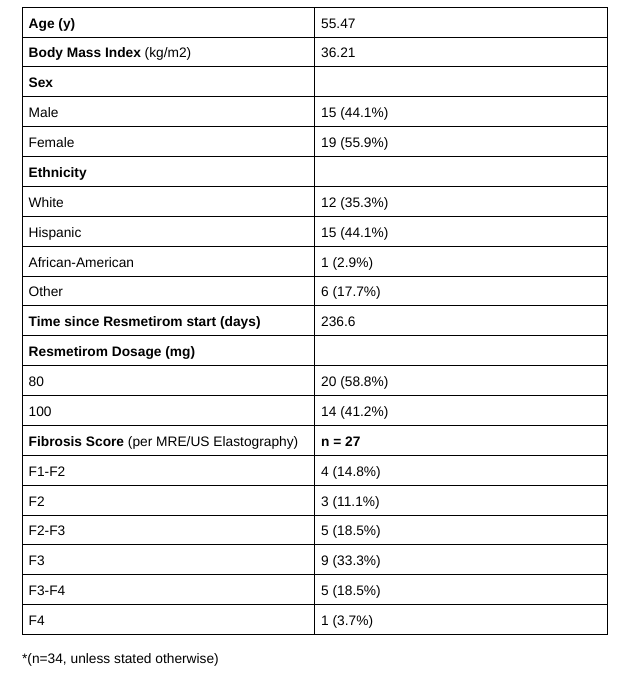
Figure: Table 1. Patient's Characteristics
Disclosures:
Dauris Rosario Lora indicated no relevant financial relationships.
Alivia Blumenstein indicated no relevant financial relationships.
Zoe Post indicated no relevant financial relationships.
Sujit Janardhan indicated no relevant financial relationships.
Nancy Reau: AbbVie – Grant/Research Support. Arbutus – Advisor or Review Panel Member. Gilead – Advisory Committee/Board Member, Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support. VIR – Advisory Committee/Board Member, Grant/Research Support.
Dauris De Jesus. Rosario Lora, MD, Alivia Blumenstein, MD, Zoe Post, MD, MSc, Sujit Janardhan, MD, PhD, Nancy Reau, MD, FACG. P5938 - Real-World Safety and Monitoring Practices in Patients Receiving Resmetirom (Rezdiffra) for MASH: A Single Center Retrospective Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Rush University Medical Center, Chicago, IL
Introduction: Resmetirom (Rezdiffra) is a selective thyroid hormone receptor-β agonist recently approved for the treatment of MASH (Metabolic dysfunction-associated steatohepatitis) with F2/3 fibrosis. While effective in clinical trials, real-world data on safety and adherence to monitoring guidelines remain limited given the medication’s recent market availability.
Methods: We conducted a retrospective analysis of patients prescribed Resmetirom at a single academic center. Data collection included demographics, treatment duration, adverse events, and adherence to laboratory monitoring guidelines, focusing on liver enzymes LFTs), fibrosis assessment and thyroid function. Patients without accessible laboratory data were excluded. A paired t-test was used for statistical analysis of NALFD Fibrosis Scores.
Results: The final sample included 34 patients. Of the initial cohort of 56 patients, 9 patients (16.1%) could not initiate therapy due to insurance denial, while the rest had either not started the drug, or had no laboratory data available. Patient characteristics are summarized in Table 1. The mean duration of therapy was 236.6 days. NAFLD fibrosis scores showed no significant change from baseline to 3–6 months (–1.17 vs. –1.21, p > 0.05), likely due to the short interval. Adverse events (Figure 1) were reported in 15 patients (44.1%), with gastrointestinal symptoms being the most common (10/15, 66.7%). Diarrhea accounted for the majority of GI complaints (6/10, 60%). There were three documented drug discontinuations, all due to diarrhea.
Monitoring practices varied. While 70% of patients had both baseline and follow-up LFTs at 3–6 months, only 38% had baseline thyroid function tests, and 14% had follow-up thyroid labs. One patient with preexisting hyperthyroidism required a levothyroxine dose adjustment after initiating therapy.
Discussion: In this real-world cohort, Resmetirom was generally well tolerated but associated with a notable rate of gastrointestinal side effects, particularly diarrhea. Monitoring practices frequently fell short of AASLD-recommended guidelines, highlighting the need for standardized protocols to ensure safety and optimize outcomes. Larger longitudinal studies are needed to better assess long-term safety and efficacy in routine clinical settings.

Figure: Figure 1. Documented Adverse Events after Resmetirom Start

Figure: Table 1. Patient's Characteristics
Disclosures:
Dauris Rosario Lora indicated no relevant financial relationships.
Alivia Blumenstein indicated no relevant financial relationships.
Zoe Post indicated no relevant financial relationships.
Sujit Janardhan indicated no relevant financial relationships.
Nancy Reau: AbbVie – Grant/Research Support. Arbutus – Advisor or Review Panel Member. Gilead – Advisory Committee/Board Member, Grant/Research Support. Salix – Advisory Committee/Board Member, Grant/Research Support. VIR – Advisory Committee/Board Member, Grant/Research Support.
Dauris De Jesus. Rosario Lora, MD, Alivia Blumenstein, MD, Zoe Post, MD, MSc, Sujit Janardhan, MD, PhD, Nancy Reau, MD, FACG. P5938 - Real-World Safety and Monitoring Practices in Patients Receiving Resmetirom (Rezdiffra) for MASH: A Single Center Retrospective Review, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
