Tuesday Poster Session
Category: Liver
P5933 - Adherence to HBV Screening and Reactivation Prevention Protocols in Patients Receiving Anti-CD20 Treatment
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Shweta Lodha, BA (she/her/hers)
Duke University School of Medicine - - Durham, NC
Roslyn, NY
Presenting Author(s)
Shweta Lodha, BA1, Kara Wegermann, MD2, Lindsay King, MD2, Andrew Muir, MD2, Jacqueline B. Henson, MD2
1Duke University School of Medicine - - Durham, NC, Durham, NC; 2Duke University School of Medicine, Durham, NC
Introduction: Anti-CD20 agents confer about a 10% risk of hepatitis B virus (HBV) reactivation in individuals with positive surface antigen (sAg) or core antibody (cAb). AGA guidelines from 2025 recommend sAg and cAb testing prior to therapy initiation. Despite these guidelines, cases of HBV reactivation due to inadequate testing or prophylaxis have occurred, underscoring the need for improved guideline adherence. This study aimed to quantify the gap in adherence to HBV testing and prophylaxis guidelines at our center, focusing on the high risk anti-CD20 agents.
Methods: We performed a single-center retrospective study of adults receiving an anti-CD20 infusion (rituximab, ocrelizumab, obintuzumab, ofatumumab) from 1/1-3/31/2024. HBV testing (sAg and cAb total) prior to anti-CD20 initiation was assessed. Use of prophylaxis in individuals with +sAg or -sAg/+cAb with tenofovir or entecavir was also assessed. The medical record of patients without apparent appropriate HBV testing or prophylaxis was reviewed to confirm diagnostic testing and treatment details. Comparisons were performed using chi square tests.
Results: A total of 803 patients received anti-CD20 infusions (rituximab=531, ocrelizumab=223, obituzumab=49, ofatumumab=2), 24% (n=189) of whom did not undergo appropriate sAg and cAb testing before initiation (Figure). The majority of those missing testing (88%; n=166) lacked cAb tests. Patients prescribed ocrelizumab were most likely to have incomplete HBV testing (Figure). Among patients without complete testing, anti-CD20 therapy was most commonly prescribed for neurologic (55%), rheumatologic (25%), or oncologic (15%) conditions. One patient was found to have +sAg and was prescribed prophylaxis. 27 patients had -sAg/+cAb, and 40% (n=13) were not prescribed prophylaxis. There were no cases of HBV reactivation during the study period.
Discussion: Nearly one quarter of patients receiving anti-CD20 therapy at a large academic medical center were not appropriately tested for HBV prior to immunosuppression initiation, and some did not receive recommended prophylaxis, placing them at risk for reactivation and fulminant liver failure. These findings highlight a critical gap in guideline adherence that would benefit from intervention. An electronic health record-based clinical decision support tool to prompt appropriate HBV testing and prophylaxis initiation upon medication ordering may help close this gap and reduce cases of HBV reactivation.
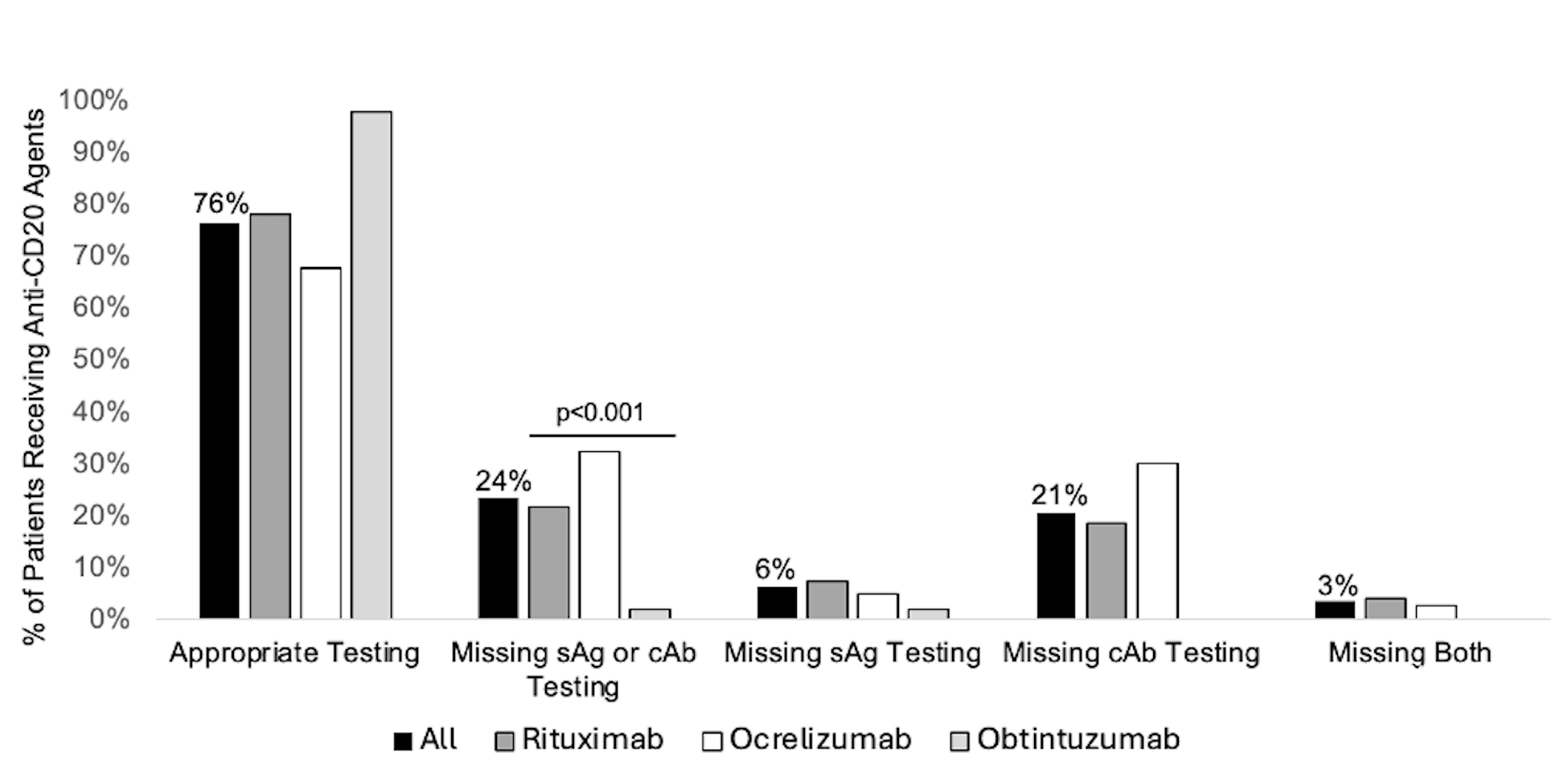
Figure: Figure. Percentage of patients receiving anti-CD20 agents with appropriate HBV testing prior to initiation, overall and by agent.
Disclosures:
Shweta Lodha indicated no relevant financial relationships.
Kara Wegermann: FocusMed CME – Speaker.
Lindsay King indicated no relevant financial relationships.
Andrew Muir: Cymabay – Grant/Research Support. Gilead – Grant/Research Support. Mirum – DSMB member. Ultragenyx – Grant/Research Support.
Jacqueline Henson indicated no relevant financial relationships.
Shweta Lodha, BA1, Kara Wegermann, MD2, Lindsay King, MD2, Andrew Muir, MD2, Jacqueline B. Henson, MD2. P5933 - Adherence to HBV Screening and Reactivation Prevention Protocols in Patients Receiving Anti-CD20 Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Duke University School of Medicine - - Durham, NC, Durham, NC; 2Duke University School of Medicine, Durham, NC
Introduction: Anti-CD20 agents confer about a 10% risk of hepatitis B virus (HBV) reactivation in individuals with positive surface antigen (sAg) or core antibody (cAb). AGA guidelines from 2025 recommend sAg and cAb testing prior to therapy initiation. Despite these guidelines, cases of HBV reactivation due to inadequate testing or prophylaxis have occurred, underscoring the need for improved guideline adherence. This study aimed to quantify the gap in adherence to HBV testing and prophylaxis guidelines at our center, focusing on the high risk anti-CD20 agents.
Methods: We performed a single-center retrospective study of adults receiving an anti-CD20 infusion (rituximab, ocrelizumab, obintuzumab, ofatumumab) from 1/1-3/31/2024. HBV testing (sAg and cAb total) prior to anti-CD20 initiation was assessed. Use of prophylaxis in individuals with +sAg or -sAg/+cAb with tenofovir or entecavir was also assessed. The medical record of patients without apparent appropriate HBV testing or prophylaxis was reviewed to confirm diagnostic testing and treatment details. Comparisons were performed using chi square tests.
Results: A total of 803 patients received anti-CD20 infusions (rituximab=531, ocrelizumab=223, obituzumab=49, ofatumumab=2), 24% (n=189) of whom did not undergo appropriate sAg and cAb testing before initiation (Figure). The majority of those missing testing (88%; n=166) lacked cAb tests. Patients prescribed ocrelizumab were most likely to have incomplete HBV testing (Figure). Among patients without complete testing, anti-CD20 therapy was most commonly prescribed for neurologic (55%), rheumatologic (25%), or oncologic (15%) conditions. One patient was found to have +sAg and was prescribed prophylaxis. 27 patients had -sAg/+cAb, and 40% (n=13) were not prescribed prophylaxis. There were no cases of HBV reactivation during the study period.
Discussion: Nearly one quarter of patients receiving anti-CD20 therapy at a large academic medical center were not appropriately tested for HBV prior to immunosuppression initiation, and some did not receive recommended prophylaxis, placing them at risk for reactivation and fulminant liver failure. These findings highlight a critical gap in guideline adherence that would benefit from intervention. An electronic health record-based clinical decision support tool to prompt appropriate HBV testing and prophylaxis initiation upon medication ordering may help close this gap and reduce cases of HBV reactivation.

Figure: Figure. Percentage of patients receiving anti-CD20 agents with appropriate HBV testing prior to initiation, overall and by agent.
Disclosures:
Shweta Lodha indicated no relevant financial relationships.
Kara Wegermann: FocusMed CME – Speaker.
Lindsay King indicated no relevant financial relationships.
Andrew Muir: Cymabay – Grant/Research Support. Gilead – Grant/Research Support. Mirum – DSMB member. Ultragenyx – Grant/Research Support.
Jacqueline Henson indicated no relevant financial relationships.
Shweta Lodha, BA1, Kara Wegermann, MD2, Lindsay King, MD2, Andrew Muir, MD2, Jacqueline B. Henson, MD2. P5933 - Adherence to HBV Screening and Reactivation Prevention Protocols in Patients Receiving Anti-CD20 Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
