Tuesday Poster Session
Category: Liver
P5968 - Sofosbuvir/Velpatasvir Causing Hepatotoxicity With Hepatocellular Pattern and Hemolytic Anemia
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- PN
Phuong Nguyen, DO
Abrazo Health Network
Chandler, AZ
Presenting Author(s)
Phuong Nguyen, DO1, Elvis Arteaga, MD2, Rohit Nathan, DO3
1Abrazo Health Network, Chandler, AZ; 2University of Arizona College of Medicine, Phoenix VA Medical Center, Phoenix, AZ; 3Banner University Medical Center, Phoenix, AZ
Introduction: A case of sofosbuvir and velpatasvir, a treatment for hepatitis C, causing hepatocellular injury and hemolytic anemia.
Case Description/
Methods: Patient is a 68 y.o. M with PMH of A-fib on Eliquis, HTN, HLD, DM, prostate cancer who was recently diagnosed with hepatitis C confirmed with liver biopsy that showed active hepatitis and stage I-II portal and periportal fibrosis who was initiated on sofosbuvir/velpatasvir 5 days prior to admission. US duplex of abdomen showed patent vasculatures. Previous Hep B core antibodies were positive and Hepatitis B DNA were less than 10, ruling out hepatitis B reactivation. MRCP showed slight prominence of the proximal CBD. Sofosbuvir/velpatasvir was continued for one day and AST increased from 967 to 1,149, ALT: 1,349 to 1,423, Bilirubin:(Conjugated predominant(4.6)) 5.9 to 6.5. When sofosbuvir/velpatasvir was discontinued, AST increased from 1,149 to 937, ALT: 1,423 to 1,104, Bilirubin: 6.5 to 6.1 over the course of two days. Baseline Hgb prior to sofosbuvir/velpatasvir was around 10 and Hgb after sofosbuvir/velpatasvir initiation was less than 7.
Discussion: This Hep C drug contains 2 active ingredients: sofosbuvir(a NS5B inhibitor) and velpatasvir (a NS5A inhibitor). In large randomized controlled trials, serum enzymes elevations were uncommon in patients treated with sofosbuvir despite the fact that the patients being treated had chronic liver disease. In multiple, large clinical trials sofosbuvir has not been linked to instances of clinically apparent liver injury with jaundice. Because sofosbuvir is always used with other antiviral agents, it is not always possible to separate the relative role of sofosbuvir from other drugs in causing adverse reactions. Two rare and unusual forms of liver injury of uncertain relationship to sofosbuvir have been described in patients with receiving antiviral therapy for hepatitis C: sudden hepatic decompensation in patients with preexisting cirrhosis and reactivation of hepatitis B in patients with preexisting evidence of HBV infection.
There are not many documented cases of sofosbuvir/velpatasvir causing direct liver injuries or hemolytic anemia beyond rare cases of hepatitis B reactivation and sudden hepatic decompensation. In this patient, both latter etiologies are ruled out with Hep B DNA levels and no clinical manifestation of abdominal ascites or hepatic encephalopathy. In this patient, liver enzymes improved quickly within 24 hours of discontinuing Sofosbuvir/velpatasvir.
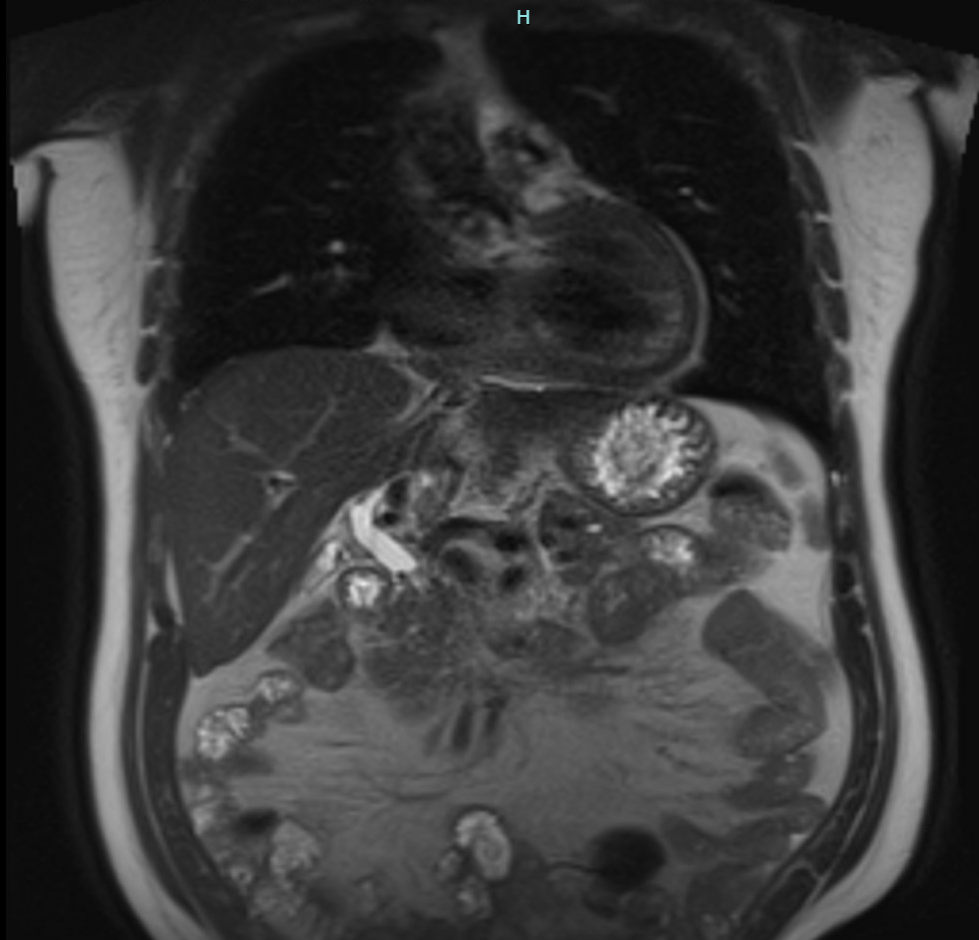
Figure: MRCP shows slight prominence of the proximal common bile duct.
Disclosures:
Phuong Nguyen indicated no relevant financial relationships.
Elvis Arteaga indicated no relevant financial relationships.
Rohit Nathan indicated no relevant financial relationships.
Phuong Nguyen, DO1, Elvis Arteaga, MD2, Rohit Nathan, DO3. P5968 - Sofosbuvir/Velpatasvir Causing Hepatotoxicity With Hepatocellular Pattern and Hemolytic Anemia, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Abrazo Health Network, Chandler, AZ; 2University of Arizona College of Medicine, Phoenix VA Medical Center, Phoenix, AZ; 3Banner University Medical Center, Phoenix, AZ
Introduction: A case of sofosbuvir and velpatasvir, a treatment for hepatitis C, causing hepatocellular injury and hemolytic anemia.
Case Description/
Methods: Patient is a 68 y.o. M with PMH of A-fib on Eliquis, HTN, HLD, DM, prostate cancer who was recently diagnosed with hepatitis C confirmed with liver biopsy that showed active hepatitis and stage I-II portal and periportal fibrosis who was initiated on sofosbuvir/velpatasvir 5 days prior to admission. US duplex of abdomen showed patent vasculatures. Previous Hep B core antibodies were positive and Hepatitis B DNA were less than 10, ruling out hepatitis B reactivation. MRCP showed slight prominence of the proximal CBD. Sofosbuvir/velpatasvir was continued for one day and AST increased from 967 to 1,149, ALT: 1,349 to 1,423, Bilirubin:(Conjugated predominant(4.6)) 5.9 to 6.5. When sofosbuvir/velpatasvir was discontinued, AST increased from 1,149 to 937, ALT: 1,423 to 1,104, Bilirubin: 6.5 to 6.1 over the course of two days. Baseline Hgb prior to sofosbuvir/velpatasvir was around 10 and Hgb after sofosbuvir/velpatasvir initiation was less than 7.
Discussion: This Hep C drug contains 2 active ingredients: sofosbuvir(a NS5B inhibitor) and velpatasvir (a NS5A inhibitor). In large randomized controlled trials, serum enzymes elevations were uncommon in patients treated with sofosbuvir despite the fact that the patients being treated had chronic liver disease. In multiple, large clinical trials sofosbuvir has not been linked to instances of clinically apparent liver injury with jaundice. Because sofosbuvir is always used with other antiviral agents, it is not always possible to separate the relative role of sofosbuvir from other drugs in causing adverse reactions. Two rare and unusual forms of liver injury of uncertain relationship to sofosbuvir have been described in patients with receiving antiviral therapy for hepatitis C: sudden hepatic decompensation in patients with preexisting cirrhosis and reactivation of hepatitis B in patients with preexisting evidence of HBV infection.
There are not many documented cases of sofosbuvir/velpatasvir causing direct liver injuries or hemolytic anemia beyond rare cases of hepatitis B reactivation and sudden hepatic decompensation. In this patient, both latter etiologies are ruled out with Hep B DNA levels and no clinical manifestation of abdominal ascites or hepatic encephalopathy. In this patient, liver enzymes improved quickly within 24 hours of discontinuing Sofosbuvir/velpatasvir.

Figure: MRCP shows slight prominence of the proximal common bile duct.
Disclosures:
Phuong Nguyen indicated no relevant financial relationships.
Elvis Arteaga indicated no relevant financial relationships.
Rohit Nathan indicated no relevant financial relationships.
Phuong Nguyen, DO1, Elvis Arteaga, MD2, Rohit Nathan, DO3. P5968 - Sofosbuvir/Velpatasvir Causing Hepatotoxicity With Hepatocellular Pattern and Hemolytic Anemia, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
