Tuesday Poster Session
Category: Liver
P5925 - Comparative Outcomes of Empagliflozin vs Dapagliflozin in Patients With Liver Cirrhosis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Imad Alabdul Razzak, MD
Phoenixville Hospital - Tower Health
Phoenixville, Pennsylvania
Presenting Author(s)
Hatem Ahmed, MD1, Sameh Gomaa, MD1, Imad Alabdul Razzak, MD1, Eyad Abdulrazzak, MBBS2
1Phoenixville Hospital - Tower Health, Phoenixville, PA; 2Beth Israel Deaconess Medical Center, Harvard Medical School, Needham, MA
Introduction: Sodium-glucose cotransporter-2 inhibitors (SGLT2i), including empagliflozin and dapagliflozin, offer cardiometabolic and renal benefits beyond glycemic control. Emerging evidence suggests they may also help manage liver cirrhosis complications such as ascites. However, comparative data between specific SGLT2 inhibitors in cirrhosis are limited. This study evaluated real-world clinical outcomes, hepatic decompensation events, and safety profiles of empagliflozin vs. dapagliflozin in cirrhosis patients.
Methods: We conducted a multicenter retrospective cohort study using the TriNetX database to identify adults with liver cirrhosis prescribed empagliflozin or dapagliflozin. Propensity score matching balanced baseline characteristics. Primary outcomes were 5-year all-cause mortality and hospitalization. Secondary outcomes included hepatic decompensation (ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding). Tertiary outcomes included hepatic, renal, and prognostic markers (albumin, bilirubin, INR, sodium). Adverse events were also assessed.
Results: Among 17,700 empagliflozin and 8,619 dapagliflozin users, 7,852 patients remained in each group after matching. No significant differences were observed in 5-year all-cause mortality (OR 1.012; 95% CI, 0.921–1.112; p = 0.81) or hospitalization. However, empagliflozin users had lower rates of hepatic encephalopathy (4.0% vs. 4.7%; OR 0.84; 95% CI, 0.717–0.983; p = 0.03), hepatorenal syndrome (1.0% vs. 1.6%; OR 0.614; 95% CI, 0.462–0.816; p < 0.001), and need for paracentesis (2.9% vs. 3.7%; OR 0.785; 95% CI, 0.656–0.94; p = 0.008). Adverse event rates were similar (Table 1).
Empagliflozin was also associated with more favorable liver function markers, including higher albumin (3.69 ± 0.72 vs. 3.61 ± 0.74; p < 0.001) and lower bilirubin (1.22 ± 2.36 vs. 1.34 ± 2.83; p = 0.009) compared to dapagliflozin (Table 2).
Discussion: In this large real-world study, empagliflozin and dapagliflozin had similar survival, hospitalization, and overall hepatic decompensation rates. However, empagliflozin was associated with lower rates of specific complications, including hepatic encephalopathy, hepatorenal syndrome, and need for paracentesis, as well as improved liver function markers, without increased adverse events. These findings suggest empagliflozin may offer targeted hepatic benefits over dapagliflozin.
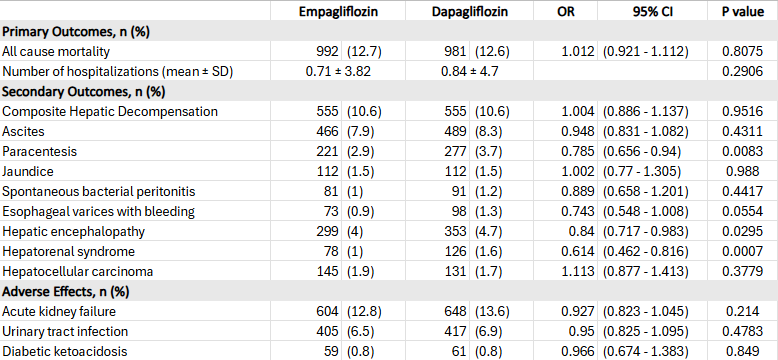
Figure: Table 1. Five-Year Outcomes in the Empagliflozin and Dapagliflozin Cohorts
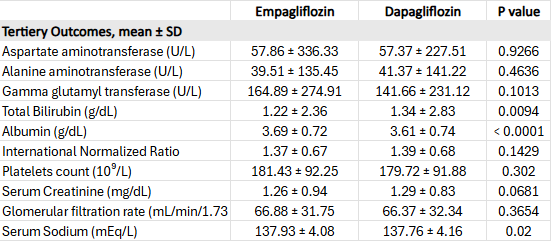
Figure: Table 2. Comparison of Hepatic and Prognostic Laboratory Parameters Between Empagliflozin and Dapagliflozin Cohorts
Disclosures:
Hatem Ahmed indicated no relevant financial relationships.
Sameh Gomaa indicated no relevant financial relationships.
Imad Alabdul Razzak indicated no relevant financial relationships.
Eyad Abdulrazzak indicated no relevant financial relationships.
Hatem Ahmed, MD1, Sameh Gomaa, MD1, Imad Alabdul Razzak, MD1, Eyad Abdulrazzak, MBBS2. P5925 - Comparative Outcomes of Empagliflozin vs Dapagliflozin in Patients With Liver Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Phoenixville Hospital - Tower Health, Phoenixville, PA; 2Beth Israel Deaconess Medical Center, Harvard Medical School, Needham, MA
Introduction: Sodium-glucose cotransporter-2 inhibitors (SGLT2i), including empagliflozin and dapagliflozin, offer cardiometabolic and renal benefits beyond glycemic control. Emerging evidence suggests they may also help manage liver cirrhosis complications such as ascites. However, comparative data between specific SGLT2 inhibitors in cirrhosis are limited. This study evaluated real-world clinical outcomes, hepatic decompensation events, and safety profiles of empagliflozin vs. dapagliflozin in cirrhosis patients.
Methods: We conducted a multicenter retrospective cohort study using the TriNetX database to identify adults with liver cirrhosis prescribed empagliflozin or dapagliflozin. Propensity score matching balanced baseline characteristics. Primary outcomes were 5-year all-cause mortality and hospitalization. Secondary outcomes included hepatic decompensation (ascites, hepatic encephalopathy, hepatorenal syndrome, variceal bleeding). Tertiary outcomes included hepatic, renal, and prognostic markers (albumin, bilirubin, INR, sodium). Adverse events were also assessed.
Results: Among 17,700 empagliflozin and 8,619 dapagliflozin users, 7,852 patients remained in each group after matching. No significant differences were observed in 5-year all-cause mortality (OR 1.012; 95% CI, 0.921–1.112; p = 0.81) or hospitalization. However, empagliflozin users had lower rates of hepatic encephalopathy (4.0% vs. 4.7%; OR 0.84; 95% CI, 0.717–0.983; p = 0.03), hepatorenal syndrome (1.0% vs. 1.6%; OR 0.614; 95% CI, 0.462–0.816; p < 0.001), and need for paracentesis (2.9% vs. 3.7%; OR 0.785; 95% CI, 0.656–0.94; p = 0.008). Adverse event rates were similar (Table 1).
Empagliflozin was also associated with more favorable liver function markers, including higher albumin (3.69 ± 0.72 vs. 3.61 ± 0.74; p < 0.001) and lower bilirubin (1.22 ± 2.36 vs. 1.34 ± 2.83; p = 0.009) compared to dapagliflozin (Table 2).
Discussion: In this large real-world study, empagliflozin and dapagliflozin had similar survival, hospitalization, and overall hepatic decompensation rates. However, empagliflozin was associated with lower rates of specific complications, including hepatic encephalopathy, hepatorenal syndrome, and need for paracentesis, as well as improved liver function markers, without increased adverse events. These findings suggest empagliflozin may offer targeted hepatic benefits over dapagliflozin.

Figure: Table 1. Five-Year Outcomes in the Empagliflozin and Dapagliflozin Cohorts

Figure: Table 2. Comparison of Hepatic and Prognostic Laboratory Parameters Between Empagliflozin and Dapagliflozin Cohorts
Disclosures:
Hatem Ahmed indicated no relevant financial relationships.
Sameh Gomaa indicated no relevant financial relationships.
Imad Alabdul Razzak indicated no relevant financial relationships.
Eyad Abdulrazzak indicated no relevant financial relationships.
Hatem Ahmed, MD1, Sameh Gomaa, MD1, Imad Alabdul Razzak, MD1, Eyad Abdulrazzak, MBBS2. P5925 - Comparative Outcomes of Empagliflozin vs Dapagliflozin in Patients With Liver Cirrhosis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
