Tuesday Poster Session
Category: Liver
P5833 - Direct Oral Anticoagulants Versus Warfarin in Elderly Patients With Child-Pugh C Cirrhosis and Atrial Fibrillation: A Real-World Perspective
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Aya Akhras, MD
HCA Florida Aventura Hospital
Aventura, FL
Presenting Author(s)
Aya Akhras, MD1, Azizullah Beran, MD2, John Guardiola, MD2, Indira Bhavsar-Burke, MD3, Bernardo Reyes, MD1, Asad Ur Rahman, MD4
1HCA Florida Aventura Hospital, Aventura, FL; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern School of Medicine, Dallas, TX; 4Cleveland Clinic Florida, Weston, FL
Introduction: Direct oral anticoagulants (DOACs) are increasingly used for stroke prevention in atrial fibrillation (AF). However, their safety in patients with advanced liver disease remains uncertain. The 2020 AASLD Practice Guidance highlights that clinical experience with DOACs in cirrhosis is primarily restricted to well-compensated cases. Furthermore, elderly patients—who are often underrepresented in clinical trials—constitute a significant proportion of those requiring hospitalization for AF and cirrhosis. This study aimed to compare clinical outcomes of DOACs versus warfarin in elderly patients with Child-Pugh C (CPC) cirrhosis and AF.
Methods: We conducted a retrospective cohort study using TriNetX, identifying patients aged ≥70 years with AF and CPC cirrhosis. Cohorts were defined based on receipt of DOACs (apixaban, rivaroxaban, dabigatran, edoxaban) or warfarin. Propensity score matching was applied to balance demographics and comorbidities. Outcomes included intracranial hemorrhage (ICH), gastrointestinal (GI) bleeding, and all-cause mortality. Descriptive statistics, Kaplan-Meier curves and Cox proportional hazards models were used. P-value < 0.05 was considered significant.
Results: A total of 1774 patients were included, with 887 in each cohort. The mean age was similar between groups (DOAC: 78.0 ± 4.8 years; Warfarin: 78.0 +/- 4.7 years), with a comparable proportion of females (DOAC: 38.9%, Warfarin: 39.7%). While differences in baseline labs, including those used to calculate MELD-Na scores, were statistically significant, they are clinically insignificant (MELD-Na: DOAC: 20.7 +/- 10.3, vs Warfarin: 22.9 +/- 11.2; p=0.029) (Table 1). The odds of GI bleeding were similar between both groups (OR 1.21, p=0.311). Compared to patients on warfarin, patients on DOACs had no difference in odds of ICH (OR 0.76, p=0.509). Lastly, there was no significant difference in odds of all cause mortality between the two groups (RR 0.96; p=0.705) (Table 2). Median survival was 205 days for the DOAC group, versus 305 days for the warfarin group (HR 1.092, p=0.082).
Discussion: In elderly patients with CPC cirrhosis and AF receiving DOAC therapy, our findings demonstrated similar odds of GI bleeding, ICH and all-cause mortality to those on warfarin therapy. Historically, the AASLD has withheld firm recommendations for DOAC use in patients with CPC due to limited evidence. These findings suggest cautious consideration of DOACs as a potential alternative to warfarin in this high-risk population.
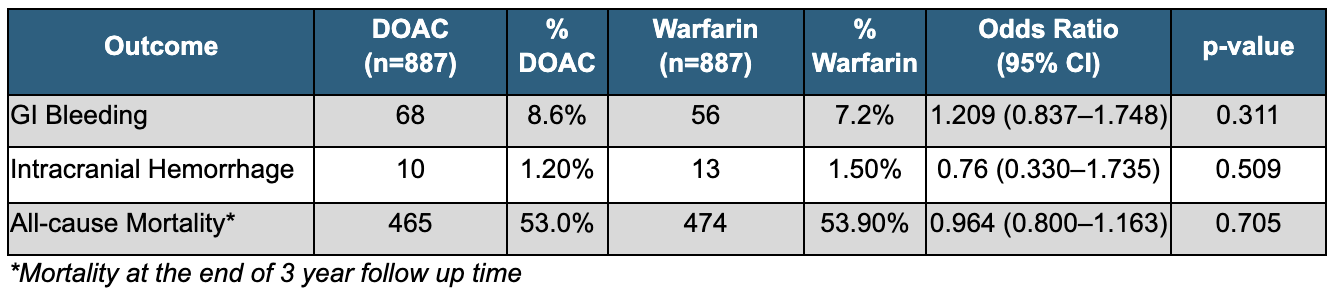
Figure: Table 1:Demographics and Baseline Characteristics of Propensity-Matched Cohorts: DOAC vs Warfarin
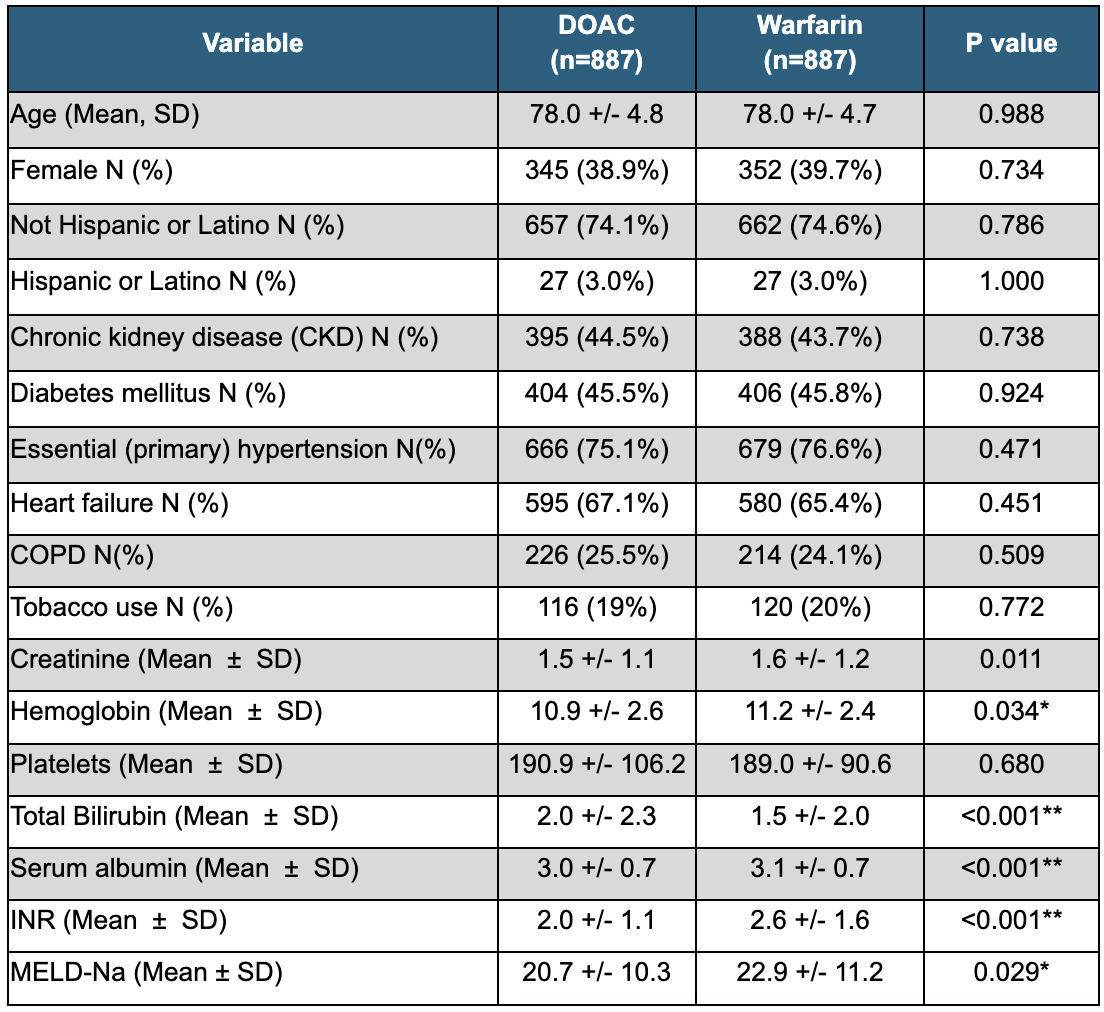
Figure: Table 2: Primary Outcomes of Anticoagulation in Elderly Patients with CPC Cirrhosis: DOAC vs Warfarin
Disclosures:
Aya Akhras indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
John Guardiola indicated no relevant financial relationships.
Indira Bhavsar-Burke indicated no relevant financial relationships.
Bernardo Reyes indicated no relevant financial relationships.
Asad Ur Rahman: Abbvie Inc – Speakers Bureau.
Aya Akhras, MD1, Azizullah Beran, MD2, John Guardiola, MD2, Indira Bhavsar-Burke, MD3, Bernardo Reyes, MD1, Asad Ur Rahman, MD4. P5833 - Direct Oral Anticoagulants Versus Warfarin in Elderly Patients With Child-Pugh C Cirrhosis and Atrial Fibrillation: A Real-World Perspective, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1HCA Florida Aventura Hospital, Aventura, FL; 2Indiana University School of Medicine, Indianapolis, IN; 3University of Texas Southwestern School of Medicine, Dallas, TX; 4Cleveland Clinic Florida, Weston, FL
Introduction: Direct oral anticoagulants (DOACs) are increasingly used for stroke prevention in atrial fibrillation (AF). However, their safety in patients with advanced liver disease remains uncertain. The 2020 AASLD Practice Guidance highlights that clinical experience with DOACs in cirrhosis is primarily restricted to well-compensated cases. Furthermore, elderly patients—who are often underrepresented in clinical trials—constitute a significant proportion of those requiring hospitalization for AF and cirrhosis. This study aimed to compare clinical outcomes of DOACs versus warfarin in elderly patients with Child-Pugh C (CPC) cirrhosis and AF.
Methods: We conducted a retrospective cohort study using TriNetX, identifying patients aged ≥70 years with AF and CPC cirrhosis. Cohorts were defined based on receipt of DOACs (apixaban, rivaroxaban, dabigatran, edoxaban) or warfarin. Propensity score matching was applied to balance demographics and comorbidities. Outcomes included intracranial hemorrhage (ICH), gastrointestinal (GI) bleeding, and all-cause mortality. Descriptive statistics, Kaplan-Meier curves and Cox proportional hazards models were used. P-value < 0.05 was considered significant.
Results: A total of 1774 patients were included, with 887 in each cohort. The mean age was similar between groups (DOAC: 78.0 ± 4.8 years; Warfarin: 78.0 +/- 4.7 years), with a comparable proportion of females (DOAC: 38.9%, Warfarin: 39.7%). While differences in baseline labs, including those used to calculate MELD-Na scores, were statistically significant, they are clinically insignificant (MELD-Na: DOAC: 20.7 +/- 10.3, vs Warfarin: 22.9 +/- 11.2; p=0.029) (Table 1). The odds of GI bleeding were similar between both groups (OR 1.21, p=0.311). Compared to patients on warfarin, patients on DOACs had no difference in odds of ICH (OR 0.76, p=0.509). Lastly, there was no significant difference in odds of all cause mortality between the two groups (RR 0.96; p=0.705) (Table 2). Median survival was 205 days for the DOAC group, versus 305 days for the warfarin group (HR 1.092, p=0.082).
Discussion: In elderly patients with CPC cirrhosis and AF receiving DOAC therapy, our findings demonstrated similar odds of GI bleeding, ICH and all-cause mortality to those on warfarin therapy. Historically, the AASLD has withheld firm recommendations for DOAC use in patients with CPC due to limited evidence. These findings suggest cautious consideration of DOACs as a potential alternative to warfarin in this high-risk population.

Figure: Table 1:Demographics and Baseline Characteristics of Propensity-Matched Cohorts: DOAC vs Warfarin

Figure: Table 2: Primary Outcomes of Anticoagulation in Elderly Patients with CPC Cirrhosis: DOAC vs Warfarin
Disclosures:
Aya Akhras indicated no relevant financial relationships.
Azizullah Beran indicated no relevant financial relationships.
John Guardiola indicated no relevant financial relationships.
Indira Bhavsar-Burke indicated no relevant financial relationships.
Bernardo Reyes indicated no relevant financial relationships.
Asad Ur Rahman: Abbvie Inc – Speakers Bureau.
Aya Akhras, MD1, Azizullah Beran, MD2, John Guardiola, MD2, Indira Bhavsar-Burke, MD3, Bernardo Reyes, MD1, Asad Ur Rahman, MD4. P5833 - Direct Oral Anticoagulants Versus Warfarin in Elderly Patients With Child-Pugh C Cirrhosis and Atrial Fibrillation: A Real-World Perspective, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
