Tuesday Poster Session
Category: Liver
P5778 - Esophagogastroduodenoscopy for Variceal Screening as Guideline Adherence Check Point in Patients Undergoing Liver Transplant Evaluation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Suria Devarapalli, DO
University of Arkansas for Medical Sciences
Little Rock, AR
Presenting Author(s)
Suria Devarapalli, DO1, Megan Schuttee, MS, BS1, John Pablo-Kaiser, BA1, Ethan Chen, BS1, Mauricio Garcia, MD1, Ragesh Thandassery, MD2
1University of Arkansas for Medical Sciences, Little Rock, AR; 2Dept of Solid Organ Transplantation, University of Arkansas for Medical Sciences, Little Rock, AR
Introduction: Pre-liver transplant (LT) esophagogastroduodenoscopy (EGD) can be a critical point to identify and ensure the compliance with ACG and AASLD guidelines on quality reporting and the use of proton pump inhibitors (PPI) and non-selective beta blockers (NSBB) in cirrhosis.
Methods: All patients undergoing LT evaluation (n=810) at a single center (Mid-West US) between Jan 2019 and Dec 2024 were analyzed. EGD reports within 6 months pre-LT were reviewed for the quality of reporting and the pattern of PPI and NSBB use before and after the procedure.
Results: Mean age was 54.6 ±10.7 years (59% males, 88.5% Caucasian, 40% obese). Etiologies of cirrhosis included alcohol (40.2%), MASLD (24.9%) and chronic hepatitis C (12.3%). Hepatocellular carcinoma (HCC) was present in 16.4%. Median MELD (IQR) at the time of initiation of LT evaluation were 18 (13-23) for the non-HCC and 11(8-15) for the HCC group.
Most EGD were performed in LT center and the primary indication was esophageal variceal (EV) screening (3.1% suspected bleeding). 23.5% EGDs were performed outside the LT center. Adherence to quality metrics such as documentation of EV, GV and PHG were acceptable (Table-1). Adherence to reporting red color signs on EV and size of GV was low. A significant proportion of reports lacked guideline-based recommendations for the follow up EGD (including those after endoscopic variceal ligation [EVL]). Pre-EGD, a low proportion of patients were on NSBBs (even after excluding those with contraindications to its use), and a large proportion of patients were on PPI without valid indication. Among those on PPI, only 40.8 % had concurrent clinical diagnosis and 3.6% had EGD findings to justify its use. Unindicated PPIs were only discontinued in 2.2% post-EGD. After prophylactic EVL, 32% were not started on NSBBs. Underutilization of NSBBs persisted even in subgroup analysis after Jan 2023 (Baveno-VII recommendations that further emphasized NSBB use were published in April 2022).
Discussion: NSBBs are underutilized in a significant proportion of patients referred to LT, both pre- and post-EGD (including those who underwent EVL). Similarly, unindicated PPI use continued post-EGD. Translating guidelines from journals to the endoscopy suite should be strongly encouraged. Endoscopists can use the EGD as a guideline adherence checkpoint to modify treatment regimen and close critical gaps in the care of patients with cirrhosis.
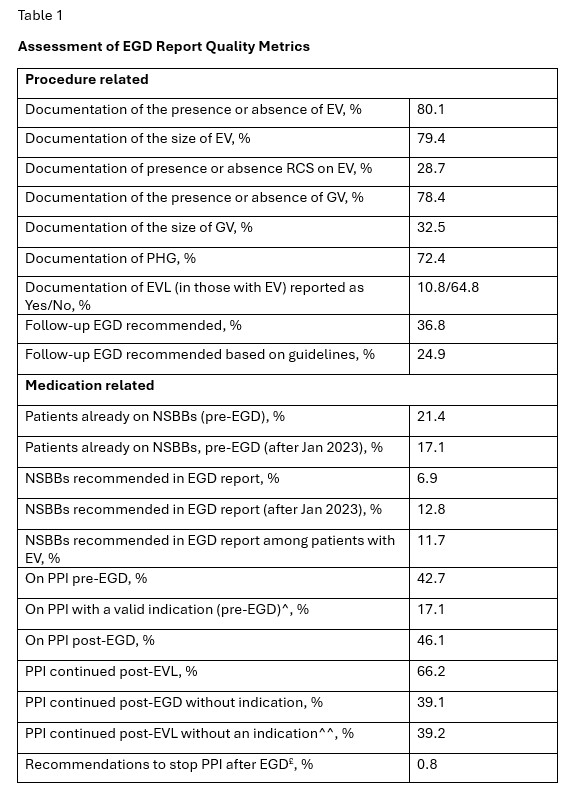
Figure: Title of Figure 1: Assessment of EGD Report Quality Metrics
Legend: EV- esophageal varices, RCS- red color signs, NSBB- nonselective betablocker, PPI- proton pump inhibitor, PHG- portal hypertensive gastropathy, ^Peptic ulcer disease, GERD, Barrett’s esophagus, eosinophilic esophagitis (among those who were on PPI), ^^ AASLD recommends stopping PPI after EVL if there is no other indication to continue its use, £ 0.8% of the whole cohort and 2.2% of those on unindicated use
Disclosures:
Suria Devarapalli indicated no relevant financial relationships.
Megan Schuttee indicated no relevant financial relationships.
John Pablo-Kaiser indicated no relevant financial relationships.
Ethan Chen indicated no relevant financial relationships.
Mauricio Garcia indicated no relevant financial relationships.
Ragesh Thandassery indicated no relevant financial relationships.
Suria Devarapalli, DO1, Megan Schuttee, MS, BS1, John Pablo-Kaiser, BA1, Ethan Chen, BS1, Mauricio Garcia, MD1, Ragesh Thandassery, MD2. P5778 - Esophagogastroduodenoscopy for Variceal Screening as Guideline Adherence Check Point in Patients Undergoing Liver Transplant Evaluation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1University of Arkansas for Medical Sciences, Little Rock, AR; 2Dept of Solid Organ Transplantation, University of Arkansas for Medical Sciences, Little Rock, AR
Introduction: Pre-liver transplant (LT) esophagogastroduodenoscopy (EGD) can be a critical point to identify and ensure the compliance with ACG and AASLD guidelines on quality reporting and the use of proton pump inhibitors (PPI) and non-selective beta blockers (NSBB) in cirrhosis.
Methods: All patients undergoing LT evaluation (n=810) at a single center (Mid-West US) between Jan 2019 and Dec 2024 were analyzed. EGD reports within 6 months pre-LT were reviewed for the quality of reporting and the pattern of PPI and NSBB use before and after the procedure.
Results: Mean age was 54.6 ±10.7 years (59% males, 88.5% Caucasian, 40% obese). Etiologies of cirrhosis included alcohol (40.2%), MASLD (24.9%) and chronic hepatitis C (12.3%). Hepatocellular carcinoma (HCC) was present in 16.4%. Median MELD (IQR) at the time of initiation of LT evaluation were 18 (13-23) for the non-HCC and 11(8-15) for the HCC group.
Most EGD were performed in LT center and the primary indication was esophageal variceal (EV) screening (3.1% suspected bleeding). 23.5% EGDs were performed outside the LT center. Adherence to quality metrics such as documentation of EV, GV and PHG were acceptable (Table-1). Adherence to reporting red color signs on EV and size of GV was low. A significant proportion of reports lacked guideline-based recommendations for the follow up EGD (including those after endoscopic variceal ligation [EVL]). Pre-EGD, a low proportion of patients were on NSBBs (even after excluding those with contraindications to its use), and a large proportion of patients were on PPI without valid indication. Among those on PPI, only 40.8 % had concurrent clinical diagnosis and 3.6% had EGD findings to justify its use. Unindicated PPIs were only discontinued in 2.2% post-EGD. After prophylactic EVL, 32% were not started on NSBBs. Underutilization of NSBBs persisted even in subgroup analysis after Jan 2023 (Baveno-VII recommendations that further emphasized NSBB use were published in April 2022).
Discussion: NSBBs are underutilized in a significant proportion of patients referred to LT, both pre- and post-EGD (including those who underwent EVL). Similarly, unindicated PPI use continued post-EGD. Translating guidelines from journals to the endoscopy suite should be strongly encouraged. Endoscopists can use the EGD as a guideline adherence checkpoint to modify treatment regimen and close critical gaps in the care of patients with cirrhosis.

Figure: Title of Figure 1: Assessment of EGD Report Quality Metrics
Legend: EV- esophageal varices, RCS- red color signs, NSBB- nonselective betablocker, PPI- proton pump inhibitor, PHG- portal hypertensive gastropathy, ^Peptic ulcer disease, GERD, Barrett’s esophagus, eosinophilic esophagitis (among those who were on PPI), ^^ AASLD recommends stopping PPI after EVL if there is no other indication to continue its use, £ 0.8% of the whole cohort and 2.2% of those on unindicated use
Disclosures:
Suria Devarapalli indicated no relevant financial relationships.
Megan Schuttee indicated no relevant financial relationships.
John Pablo-Kaiser indicated no relevant financial relationships.
Ethan Chen indicated no relevant financial relationships.
Mauricio Garcia indicated no relevant financial relationships.
Ragesh Thandassery indicated no relevant financial relationships.
Suria Devarapalli, DO1, Megan Schuttee, MS, BS1, John Pablo-Kaiser, BA1, Ethan Chen, BS1, Mauricio Garcia, MD1, Ragesh Thandassery, MD2. P5778 - Esophagogastroduodenoscopy for Variceal Screening as Guideline Adherence Check Point in Patients Undergoing Liver Transplant Evaluation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

