Tuesday Poster Session
Category: Liver
P5774 - No Significant Differences in Risk of Cirrhosis or Overall Mortality Between Untreated Immune Tolerant vs Treated Immune Active Chronic Hepatitis B Patients
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- ZY
Zeyuan Yang, MS
Palo Alto Veterans Institute for Research
Palo Alto, CA
Presenting Author(s)
Zeyuan Yang, MS1, Ramsey Cheung, MD2, Robert Wong, MD, MS, FACG2
1Palo Alto Veterans Institute for Research, Palo Alto, CA; 2Stanford University School of Medicine, Palo Alto, CA
Introduction: While most existing guidelines do not recommend treatment of chronic hepatitis B (CHB) patients in the immune tolerant phase, there are emerging data that risks of disease progression in untreated immune tolerant (U-IT) are not negligible and in some cases, this risk is higher than treated immune active (T-IA) CHB patients. However, most of these studies have been conducted in Asian countries, and few data in Western populations are available. We aim to evaluate long-term risks of cirrhosis and overall mortality between U-IT vs. T-IA CHB patients among a national cohort of U.S. Veterans.
Methods: Using national Veterans Affairs data, we identified adults with CHB (minimum >1 year follow-up) that met criteria for U-IT or T-IA from 1/1/2010 to 12/31/2021 (with follow-up through 12/31/2024). Patients with concurrent HIV, HCV, HDV, or prior cirrhosis or HCC diagnosis were excluded. Propensity score weighting (PSW) methods were applied to balance baseline factors. Primary outcomes were development of cirrhosis or death/liver transplant [LT] (censoring event 6 months after start of antiviral therapy in U-IT or end of the study period). Incidence of cirrhosis or death/LT (per 100 person-years [py]) was compared between U-IT and T-IA, and Cox proportional hazards regression models evaluated differences in risk of cirrhosis or death/LT between groups.
Results: Among 591 U-IT and 555 T-IA CHB patients identified, baseline characteristics were similar after PSW (mean age 56.4-57.5 years, 45-50% non-Hispanic white, 37-40% African American, 9-10% Asian, 25-29% concurrent diabetes, 35-39% with FIB-4 > 3.25). Over a median follow-up of 6.4 years (IQR 3.0-10.4), the incidence of cirrhosis or death/LT was similar between T-IA vs. U-IT groups (cirrhosis: 3.79 [95% CI 3.39-4.25] vs. 3.64 [95% CI 3.23-4.10] per 100 py, p=0.62; death/LT: 3.47 [95% CI 3.08-3.91] vs. 3.59 [95% CI 3.18-4.04] per 100 py, p=0.70) (Figure). On Cox proportional hazards regression, when compared to T-IA CHB, no difference in risk of cirrhosis or death/LT was observed in U-IT CHB patients (cirrhosis: HR 0.95, 95% CI 0.80-1.10, p=0.44; death/LT: HR 1.04, 95% CI 0.88-1.23, p=0.65).
Discussion: Among a longitudinal national cohort of Veterans with CHB, no significant differences in long-term risks of cirrhosis or death/LT was observed between U-IT vs. T-IA patients. Antiviral therapy in IA CHB patients is effective in reducing risks of cirrhosis or death/LT to levels similar to U-IT patients.
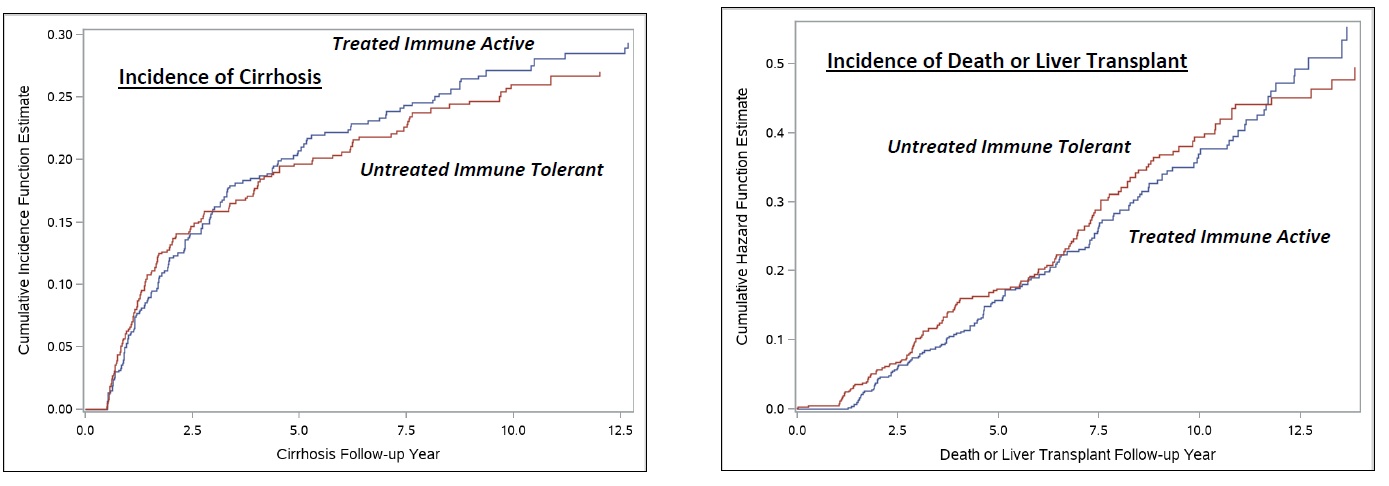
Figure: Incidence of Cirrhosis or Death/Liver Transplant Between Untreated Immune Tolerant vs. Treated Immune Active Chronic Hepatitis B Patients.
Disclosures:
Zeyuan Yang indicated no relevant financial relationships.
Ramsey Cheung indicated no relevant financial relationships.
Robert Wong: Durect Corporation – Grant/Research Support. Exact Sciences – Grant/Research Support. Gilead Sciences – Grant/Research Support. Thera Technologies – Grant/Research Support.
Zeyuan Yang, MS1, Ramsey Cheung, MD2, Robert Wong, MD, MS, FACG2. P5774 - No Significant Differences in Risk of Cirrhosis or Overall Mortality Between Untreated Immune Tolerant vs Treated Immune Active Chronic Hepatitis B Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Palo Alto Veterans Institute for Research, Palo Alto, CA; 2Stanford University School of Medicine, Palo Alto, CA
Introduction: While most existing guidelines do not recommend treatment of chronic hepatitis B (CHB) patients in the immune tolerant phase, there are emerging data that risks of disease progression in untreated immune tolerant (U-IT) are not negligible and in some cases, this risk is higher than treated immune active (T-IA) CHB patients. However, most of these studies have been conducted in Asian countries, and few data in Western populations are available. We aim to evaluate long-term risks of cirrhosis and overall mortality between U-IT vs. T-IA CHB patients among a national cohort of U.S. Veterans.
Methods: Using national Veterans Affairs data, we identified adults with CHB (minimum >1 year follow-up) that met criteria for U-IT or T-IA from 1/1/2010 to 12/31/2021 (with follow-up through 12/31/2024). Patients with concurrent HIV, HCV, HDV, or prior cirrhosis or HCC diagnosis were excluded. Propensity score weighting (PSW) methods were applied to balance baseline factors. Primary outcomes were development of cirrhosis or death/liver transplant [LT] (censoring event 6 months after start of antiviral therapy in U-IT or end of the study period). Incidence of cirrhosis or death/LT (per 100 person-years [py]) was compared between U-IT and T-IA, and Cox proportional hazards regression models evaluated differences in risk of cirrhosis or death/LT between groups.
Results: Among 591 U-IT and 555 T-IA CHB patients identified, baseline characteristics were similar after PSW (mean age 56.4-57.5 years, 45-50% non-Hispanic white, 37-40% African American, 9-10% Asian, 25-29% concurrent diabetes, 35-39% with FIB-4 > 3.25). Over a median follow-up of 6.4 years (IQR 3.0-10.4), the incidence of cirrhosis or death/LT was similar between T-IA vs. U-IT groups (cirrhosis: 3.79 [95% CI 3.39-4.25] vs. 3.64 [95% CI 3.23-4.10] per 100 py, p=0.62; death/LT: 3.47 [95% CI 3.08-3.91] vs. 3.59 [95% CI 3.18-4.04] per 100 py, p=0.70) (Figure). On Cox proportional hazards regression, when compared to T-IA CHB, no difference in risk of cirrhosis or death/LT was observed in U-IT CHB patients (cirrhosis: HR 0.95, 95% CI 0.80-1.10, p=0.44; death/LT: HR 1.04, 95% CI 0.88-1.23, p=0.65).
Discussion: Among a longitudinal national cohort of Veterans with CHB, no significant differences in long-term risks of cirrhosis or death/LT was observed between U-IT vs. T-IA patients. Antiviral therapy in IA CHB patients is effective in reducing risks of cirrhosis or death/LT to levels similar to U-IT patients.

Figure: Incidence of Cirrhosis or Death/Liver Transplant Between Untreated Immune Tolerant vs. Treated Immune Active Chronic Hepatitis B Patients.
Disclosures:
Zeyuan Yang indicated no relevant financial relationships.
Ramsey Cheung indicated no relevant financial relationships.
Robert Wong: Durect Corporation – Grant/Research Support. Exact Sciences – Grant/Research Support. Gilead Sciences – Grant/Research Support. Thera Technologies – Grant/Research Support.
Zeyuan Yang, MS1, Ramsey Cheung, MD2, Robert Wong, MD, MS, FACG2. P5774 - No Significant Differences in Risk of Cirrhosis or Overall Mortality Between Untreated Immune Tolerant vs Treated Immune Active Chronic Hepatitis B Patients, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
