Tuesday Poster Session
Category: Liver
P5765 - Impact of Glucagon-Like Peptide-1 (GLP-1) Analogues Use on Weight Trajectory and Clinical Outcomes Following Liver Transplantation
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Muhammad Saad Faisal, MD (he/him/his)
Henry Ford Health
Detroit, MI
Presenting Author(s)
Muhammad Saad Faisal, MD1, Gokturk Tepe, 2, Bradley Karmo, MD3, Agustin Gavidia Rosario, MD1, Ingrid Rocha, BS4, Minahil Fatima, MD1, Muhammad Salman Faisal, MD5, Syed-Mohammed Jafri, MD1
1Henry Ford Health, Detroit, MI; 2Wayne State University, Detroit, MI; 3Henry Ford Health, West Bloomfield, MI; 4Wayne State School of Medicine, Detroit, MI; 5Henry Ford Hospital, Detroit, MI
Introduction: Glucagon-Like Peptide-1 (GLP-1) analogues are increasingly being used for the management of diabetes and obesity. However, their use in liver transplant (LT) recipients remains understudied, particularly regarding safety, rejection risk, and impact on post-transplant weight gain. This study aimed to evaluate the clinical characteristics and outcomes associated with GLP-1 use following LT.
Methods: We conducted a retrospective cohort study of adult LT recipients between 2019-2023 at a single tertiary care center. Patients were divided into two groups based on GLP-1 use after transplant. Baseline demographics, metabolic comorbidities, transplant characteristics, and post-transplant outcomes were compared. Categorical variables were analyzed using Chi-square tests, and continuous variables using t-tests.
Results: Among 368 LT recipients, 73 (19.8%) were prescribed GLP-1 analogues post-transplant and 295(80.2%) were not. There was no difference in demographics between the 2 groups. GLP-1 users had significantly higher rates of diabetes (79.5% vs. 31.2%, p< 0.0001), hyperlipidemia (50.7% vs. 29.8%, p=0.0012), and BMI at transplant (31.9 ± 6.3 vs. 28.5 ± 12.6, p=0.0015). MASH was the leading indication for transplant in the GLP-1 group (61.9% vs. 38.1%, p< 0.0001). There were no significant differences in ischemia times, or length of hospital stay. GLP-1 was initiated at a mean of 28.3 ± 19.9 months post-transplant. At year 1, GLP-1 users had lower rejection rates (12.3% vs. 23.1%, p=0.0635), with a similar trend at 3 years (6.8% vs. 9.5%, p=0.6322). Mortality was low in both groups; at 3 years, mortality was lower in GLP-1 users (1.4% vs. 4.7%, p=0.3293). Delta BMI at 1 year (−0.61 vs. +0.91) and 3 years was not significantly different.
Discussion: GLP-1 analogues were commonly used post-LT in patients with MASH and obesity. Despite higher metabolic burden at baseline, GLP-1 use was associated with similar or improved trends in BMI, rejection, and mortality, supporting their safety in the post-transplant population. Larger prospective studies are needed to validate these findings and to strengthen the evidence supporting the use of GLP-1 analogues in this high-risk population.
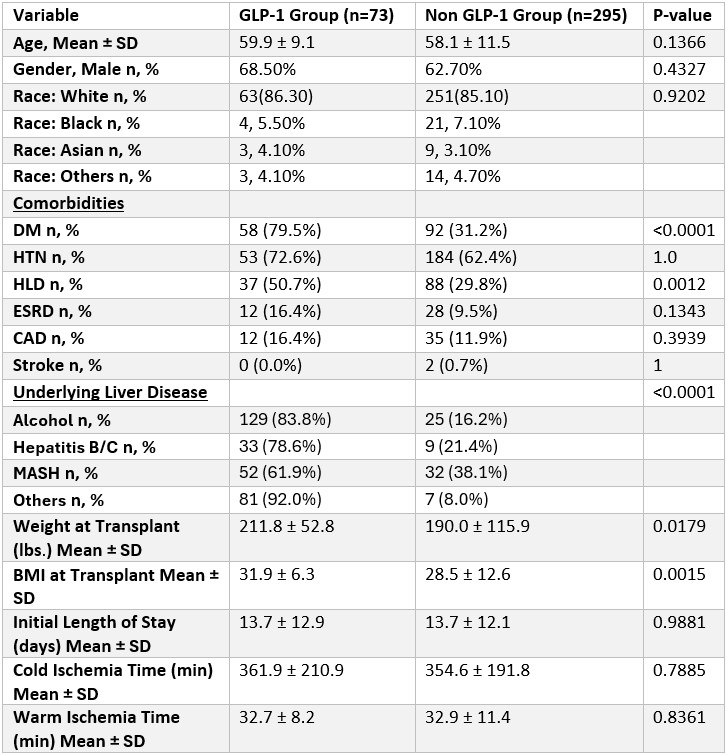
Figure: Table 1. Comparison of demographics, comorbidities, underlying liver disease and transplant characteristics.
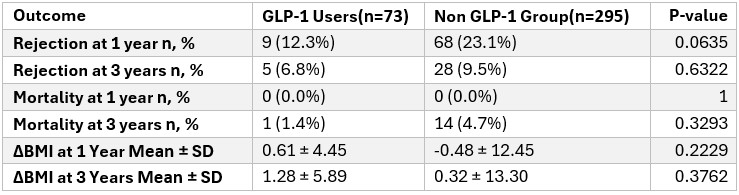
Figure: Table 2. Comparison of Outcomes
Disclosures:
Muhammad Saad Faisal indicated no relevant financial relationships.
Gokturk Tepe indicated no relevant financial relationships.
Bradley Karmo indicated no relevant financial relationships.
Agustin Gavidia Rosario indicated no relevant financial relationships.
Ingrid Rocha indicated no relevant financial relationships.
Minahil Fatima indicated no relevant financial relationships.
Muhammad Salman Faisal indicated no relevant financial relationships.
Syed-Mohammed Jafri: Abbvie – Speakers Bureau. Gilead – Speakers Bureau. Intercept – Speakers Bureau. Ironwood – Speakers Bureau. Takeda – Speakers Bureau.
Muhammad Saad Faisal, MD1, Gokturk Tepe, 2, Bradley Karmo, MD3, Agustin Gavidia Rosario, MD1, Ingrid Rocha, BS4, Minahil Fatima, MD1, Muhammad Salman Faisal, MD5, Syed-Mohammed Jafri, MD1. P5765 - Impact of Glucagon-Like Peptide-1 (GLP-1) Analogues Use on Weight Trajectory and Clinical Outcomes Following Liver Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Henry Ford Health, Detroit, MI; 2Wayne State University, Detroit, MI; 3Henry Ford Health, West Bloomfield, MI; 4Wayne State School of Medicine, Detroit, MI; 5Henry Ford Hospital, Detroit, MI
Introduction: Glucagon-Like Peptide-1 (GLP-1) analogues are increasingly being used for the management of diabetes and obesity. However, their use in liver transplant (LT) recipients remains understudied, particularly regarding safety, rejection risk, and impact on post-transplant weight gain. This study aimed to evaluate the clinical characteristics and outcomes associated with GLP-1 use following LT.
Methods: We conducted a retrospective cohort study of adult LT recipients between 2019-2023 at a single tertiary care center. Patients were divided into two groups based on GLP-1 use after transplant. Baseline demographics, metabolic comorbidities, transplant characteristics, and post-transplant outcomes were compared. Categorical variables were analyzed using Chi-square tests, and continuous variables using t-tests.
Results: Among 368 LT recipients, 73 (19.8%) were prescribed GLP-1 analogues post-transplant and 295(80.2%) were not. There was no difference in demographics between the 2 groups. GLP-1 users had significantly higher rates of diabetes (79.5% vs. 31.2%, p< 0.0001), hyperlipidemia (50.7% vs. 29.8%, p=0.0012), and BMI at transplant (31.9 ± 6.3 vs. 28.5 ± 12.6, p=0.0015). MASH was the leading indication for transplant in the GLP-1 group (61.9% vs. 38.1%, p< 0.0001). There were no significant differences in ischemia times, or length of hospital stay. GLP-1 was initiated at a mean of 28.3 ± 19.9 months post-transplant. At year 1, GLP-1 users had lower rejection rates (12.3% vs. 23.1%, p=0.0635), with a similar trend at 3 years (6.8% vs. 9.5%, p=0.6322). Mortality was low in both groups; at 3 years, mortality was lower in GLP-1 users (1.4% vs. 4.7%, p=0.3293). Delta BMI at 1 year (−0.61 vs. +0.91) and 3 years was not significantly different.
Discussion: GLP-1 analogues were commonly used post-LT in patients with MASH and obesity. Despite higher metabolic burden at baseline, GLP-1 use was associated with similar or improved trends in BMI, rejection, and mortality, supporting their safety in the post-transplant population. Larger prospective studies are needed to validate these findings and to strengthen the evidence supporting the use of GLP-1 analogues in this high-risk population.

Figure: Table 1. Comparison of demographics, comorbidities, underlying liver disease and transplant characteristics.

Figure: Table 2. Comparison of Outcomes
Disclosures:
Muhammad Saad Faisal indicated no relevant financial relationships.
Gokturk Tepe indicated no relevant financial relationships.
Bradley Karmo indicated no relevant financial relationships.
Agustin Gavidia Rosario indicated no relevant financial relationships.
Ingrid Rocha indicated no relevant financial relationships.
Minahil Fatima indicated no relevant financial relationships.
Muhammad Salman Faisal indicated no relevant financial relationships.
Syed-Mohammed Jafri: Abbvie – Speakers Bureau. Gilead – Speakers Bureau. Intercept – Speakers Bureau. Ironwood – Speakers Bureau. Takeda – Speakers Bureau.
Muhammad Saad Faisal, MD1, Gokturk Tepe, 2, Bradley Karmo, MD3, Agustin Gavidia Rosario, MD1, Ingrid Rocha, BS4, Minahil Fatima, MD1, Muhammad Salman Faisal, MD5, Syed-Mohammed Jafri, MD1. P5765 - Impact of Glucagon-Like Peptide-1 (GLP-1) Analogues Use on Weight Trajectory and Clinical Outcomes Following Liver Transplantation, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
