Tuesday Poster Session
Category: Infections and Microbiome
P5555 - Using Oral Capsules for FMT Is as Safe and Effective for Recurrent Clostridium Difficile Infection : A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Ashesh Das, MBBS
KPC Medical College and Hospital , Kolkata, India
Kolkata, West Bengal, India
Presenting Author(s)
Ashesh Das, MBBS1, Fazia Khattak, 2, Venkata Dileep Kumar Veldi, MBBS3, Emil Vergis Philip, MBBS4, Prishita Banerji, MD5, Sarthak H. Dhruv, MBBS6, Akash Rawat, MD7, Saisravika Kambham, MBBS8, Anchit Chauhan, 9, Jay Prakashbhai. Dankhara, MD10, Thangeti Dheeraja Phani, MBBS11, Karma Patel, MBBS12, Aditi Yadav, MBBS13, Ishani Mishra, MBBS14
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 3Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 4Madras Medical College, Kottayam, Kerala, India; 5University of Nis Faculty of Medicine, Lucknow, Uttar Pradesh, India; 6C. U. Shah Medical College, Rajkot, Gujarat, India; 7Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun, Uttarakhand, India; 8Dr B.R. Ambedkar Medical College and Hospital, Troy, MI; 9Maulana Azad Medical College, New Delhi, Delhi, India; 10International Higher School of Medicine, Surat, Gujarat, India; 11Alluri Sitarama Raju Institute of Medical Sciences, Rajahmahendravaram, Andhra Pradesh, India; 12GMERS Medical College and Hospital, Himmatnagar, Gujarat, India; 13Adesh Medical College and Hospital, Kurukshetra, Haryana, India; 14Bharati Vidyapeeth Medical College, Pune, Delhi, Delhi, India
Introduction: Recurrent Clostridioides difficile infection (rCDI) poses a significant challenge for clinicians and is the primary indication for faecal microbiota transplantation (FMT), increasing U.S. healthcare spending by over $1 billion. Yet most centres still rely on colonoscopy or enema delivery for FMT—procedures that are invasive, resource-intensive, and poorly tolerated. Oral, freeze-dried FMT capsules promise guideline-shifting convenience, but concerns persist that capsule transit or gastric acidity might blunt efficacy or raise safety risks.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing oral FMT Capsules in comparison to freeze dried FMT via enema or placebo upto the large randomized MATCH 2025 study. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: In three eligible RCTs (n = 323; oral-capsule FMT = 160, comparators = 163) adverse-event, mortality, and recurrence rates were indistinguishable between delivery routes. Any treatment-related adverse event occurred in 13.1 % of capsule recipients’ vs 16.0 % of controls (RR 0.83, 95 % CI 0.50–1.38; P = 0.47; I² = 0 %), with no effect-modification when trials were stratified by active-route vs placebo comparator (interaction P = 0.92). All-cause mortality was numerically lower with capsules (1.9 % vs 3.7 %; RR 0.71, 0.22–2.32), though precision was limited and heterogeneity modest (I² = 37 %; interaction P = 0.09). Recurrent C. difficile infection ≤8 weeks occurred in 8.1 % vs 8.0 % (RR 1.04, 0.50–2.15; I² = 0 %), again with no subgroup signal (interaction P = 0.64).
Discussion: Capsule FMT was non-inferior to invasive delivery or placebo in rCDI, showing identical recurrence rates and no increase in adverse events or mortality. The zero to low heterogeneity and consistent subgroup signals confirm that convenience does not come at the cost of efficacy or safety. Adopting capsules could spare patients procedural risks, slash endoscopy-suite burden, and broaden FMT access—particularly in outpatient or resource-limited settings. Large pragmatic trials and cost-effectiveness analyses should now focus on how to best integrate capsule FMT into patient care pathways.
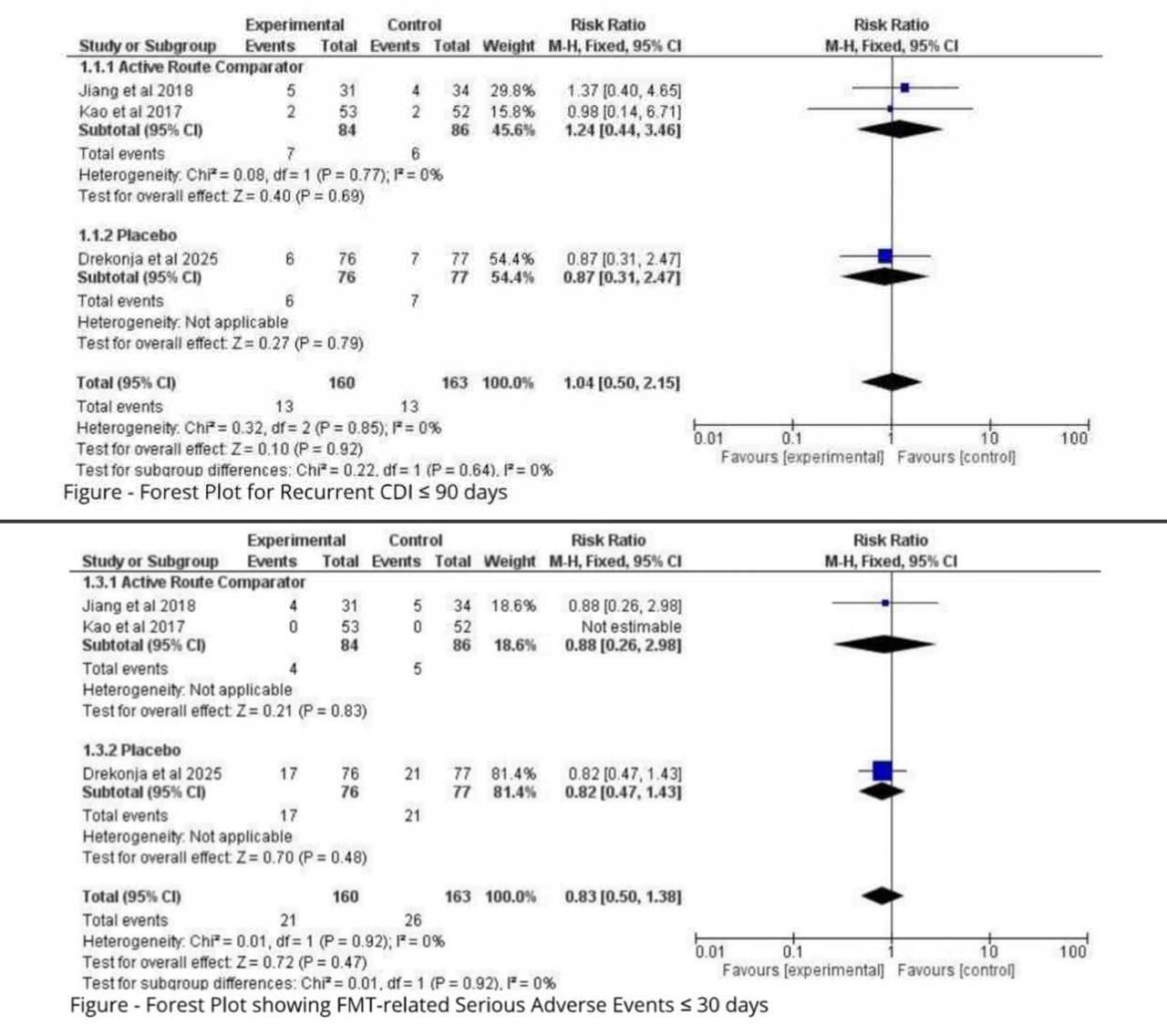
Figure: Figure Showing Forest Plots for Recurrent CDI within 90 Days Comparing Oral Freeze-Dried FMT with Enema or Placebo, and FMT-Related Adverse Effects within 30 Days
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Emil Vergis Philip indicated no relevant financial relationships.
Prishita Banerji indicated no relevant financial relationships.
Sarthak Dhruv indicated no relevant financial relationships.
Akash Rawat indicated no relevant financial relationships.
Saisravika Kambham indicated no relevant financial relationships.
Anchit Chauhan indicated no relevant financial relationships.
Jay Dankhara indicated no relevant financial relationships.
Thangeti Dheeraja Phani indicated no relevant financial relationships.
Karma Patel indicated no relevant financial relationships.
Aditi Yadav indicated no relevant financial relationships.
Ishani Mishra indicated no relevant financial relationships.
Ashesh Das, MBBS1, Fazia Khattak, 2, Venkata Dileep Kumar Veldi, MBBS3, Emil Vergis Philip, MBBS4, Prishita Banerji, MD5, Sarthak H. Dhruv, MBBS6, Akash Rawat, MD7, Saisravika Kambham, MBBS8, Anchit Chauhan, 9, Jay Prakashbhai. Dankhara, MD10, Thangeti Dheeraja Phani, MBBS11, Karma Patel, MBBS12, Aditi Yadav, MBBS13, Ishani Mishra, MBBS14. P5555 - Using Oral Capsules for FMT Is as Safe and Effective for Recurrent Clostridium Difficile Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1KPC Medical College and Hospital , Kolkata, India, Kolkata, West Bengal, India; 2Khyber Medical College, Peshawar, North-West Frontier, Pakistan; 3Gayatri Vidya Parishad Institute of Health care and Medical Technology, Visakhapatnam, Andhra Pradesh, India; 4Madras Medical College, Kottayam, Kerala, India; 5University of Nis Faculty of Medicine, Lucknow, Uttar Pradesh, India; 6C. U. Shah Medical College, Rajkot, Gujarat, India; 7Himalayan Institute of Medical Sciences, Swami Rama Himalayan University, Dehradun, Uttarakhand, India; 8Dr B.R. Ambedkar Medical College and Hospital, Troy, MI; 9Maulana Azad Medical College, New Delhi, Delhi, India; 10International Higher School of Medicine, Surat, Gujarat, India; 11Alluri Sitarama Raju Institute of Medical Sciences, Rajahmahendravaram, Andhra Pradesh, India; 12GMERS Medical College and Hospital, Himmatnagar, Gujarat, India; 13Adesh Medical College and Hospital, Kurukshetra, Haryana, India; 14Bharati Vidyapeeth Medical College, Pune, Delhi, Delhi, India
Introduction: Recurrent Clostridioides difficile infection (rCDI) poses a significant challenge for clinicians and is the primary indication for faecal microbiota transplantation (FMT), increasing U.S. healthcare spending by over $1 billion. Yet most centres still rely on colonoscopy or enema delivery for FMT—procedures that are invasive, resource-intensive, and poorly tolerated. Oral, freeze-dried FMT capsules promise guideline-shifting convenience, but concerns persist that capsule transit or gastric acidity might blunt efficacy or raise safety risks.
Methods: A systematic search of PubMed, Embase, Scopus, and Cochrane Library identified Randomized Controlled Trials (RCTs) comparing oral FMT Capsules in comparison to freeze dried FMT via enema or placebo upto the large randomized MATCH 2025 study. Data were analysed using RevMan 4.2.1. Pooled risk ratios (RRs) with 95% confidence intervals (CIs) were calculated using Mantel-Haenszel methods. Random- or fixed-effects models were applied based on heterogeneity (Higgins’ I²). Statistical significance was set at p < 0.05. Risk of bias was assessed using RoB 2.0.
Results: In three eligible RCTs (n = 323; oral-capsule FMT = 160, comparators = 163) adverse-event, mortality, and recurrence rates were indistinguishable between delivery routes. Any treatment-related adverse event occurred in 13.1 % of capsule recipients’ vs 16.0 % of controls (RR 0.83, 95 % CI 0.50–1.38; P = 0.47; I² = 0 %), with no effect-modification when trials were stratified by active-route vs placebo comparator (interaction P = 0.92). All-cause mortality was numerically lower with capsules (1.9 % vs 3.7 %; RR 0.71, 0.22–2.32), though precision was limited and heterogeneity modest (I² = 37 %; interaction P = 0.09). Recurrent C. difficile infection ≤8 weeks occurred in 8.1 % vs 8.0 % (RR 1.04, 0.50–2.15; I² = 0 %), again with no subgroup signal (interaction P = 0.64).
Discussion: Capsule FMT was non-inferior to invasive delivery or placebo in rCDI, showing identical recurrence rates and no increase in adverse events or mortality. The zero to low heterogeneity and consistent subgroup signals confirm that convenience does not come at the cost of efficacy or safety. Adopting capsules could spare patients procedural risks, slash endoscopy-suite burden, and broaden FMT access—particularly in outpatient or resource-limited settings. Large pragmatic trials and cost-effectiveness analyses should now focus on how to best integrate capsule FMT into patient care pathways.

Figure: Figure Showing Forest Plots for Recurrent CDI within 90 Days Comparing Oral Freeze-Dried FMT with Enema or Placebo, and FMT-Related Adverse Effects within 30 Days
Disclosures:
Ashesh Das indicated no relevant financial relationships.
Fazia Khattak indicated no relevant financial relationships.
Venkata Dileep Kumar Veldi indicated no relevant financial relationships.
Emil Vergis Philip indicated no relevant financial relationships.
Prishita Banerji indicated no relevant financial relationships.
Sarthak Dhruv indicated no relevant financial relationships.
Akash Rawat indicated no relevant financial relationships.
Saisravika Kambham indicated no relevant financial relationships.
Anchit Chauhan indicated no relevant financial relationships.
Jay Dankhara indicated no relevant financial relationships.
Thangeti Dheeraja Phani indicated no relevant financial relationships.
Karma Patel indicated no relevant financial relationships.
Aditi Yadav indicated no relevant financial relationships.
Ishani Mishra indicated no relevant financial relationships.
Ashesh Das, MBBS1, Fazia Khattak, 2, Venkata Dileep Kumar Veldi, MBBS3, Emil Vergis Philip, MBBS4, Prishita Banerji, MD5, Sarthak H. Dhruv, MBBS6, Akash Rawat, MD7, Saisravika Kambham, MBBS8, Anchit Chauhan, 9, Jay Prakashbhai. Dankhara, MD10, Thangeti Dheeraja Phani, MBBS11, Karma Patel, MBBS12, Aditi Yadav, MBBS13, Ishani Mishra, MBBS14. P5555 - Using Oral Capsules for FMT Is as Safe and Effective for Recurrent Clostridium Difficile Infection: A Systematic Review and Meta-Analysis of Randomized Controlled Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
