Tuesday Poster Session
Category: IBD
P5549 - Vedolizumab-Associated Acute Pancreatitis After 5 Years of Use in a Patient With Ulcerative Colitis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
.jpg)
Wei Tang, MD
Westchester Medical Center
Valhalla, NY
Presenting Author(s)
Wei Tang, MD1, Deepali Tewari, MD2, Maxwell Charlat, MD1, Virendra Tewari, MBBS, MD, DM, FACG2
1Westchester Medical Center, Valhalla, NY; 2New York Medical College, Valhalla, NY
Introduction: Vedolizumab (VDZ), an α4β7 integrin inhibitor, is widely used in moderate-to-severe inflammatory bowel disease (IBD) due to its gut-selective action and favorable safety profile. Drug-induced acute pancreatitis (DIAP) is rare. We present a case of VDZ-associated acute pancreatitis (AP) in a patient with ulcerative colitis (UC) after 5 years of therapy.
Case Description/
Methods: A 32-year-old woman with left-sided UC (diagnosed 7 years ago, extending to the splenic flexure) and antiphospholipid syndrome (APLS) on aspirin for thromboprophylaxis presented with 4 days of severe, stabbing epigastric pain radiating to the back. She had ischemic colitis of the hepatic flexure 11 years ago and subsequent workup revealed APLS. She had been in clinical and endoscopic remission on VDZ infusions every 8 weeks for 5 years; her next dose was due in 4 days.
Initial labs (Table 1) showed elevated lipase (420 U/L) and amylase (389 U/L), with normal inflammatory markers and no signs of organ failure. She was hospitalized for 3 days for significant pain. Esophagogastroduodenoscopy (EGD) was unremarkable and upper endoscopic ultrasound (EUS) revealed normal appearing pancreas, common bile duct, and gallbladder. CT angiography showed normal vasculature. Magnetic resonance cholangiopancreatography (MRCP) revealed pancreatic head and tail edema, consistent with pancreatitis (Fig. 1). Further evaluation—including lipid panel, IgG4 level, and hereditary pancreatitis genetic panel—was unremarkable. With no other identifiable cause, DIAP was suspected.
Over 3-4 weeks her lipase and amylase were still elevated, but the inflammatory markers remained normal, and pain gradually improved. Her VDZ was delayed, and she opted to switch to subcutaneous formulation, which she tolerated without worsening pain.
Discussion: DIAP accounts for < 5% of AP and is a diagnosis of exclusion. A 2023 analysis of Takeda’s Global Safety Database ( >700,000 patient-years) reported only196 cases of pancreatitis in VDZ-treated IBD patients, almost all were serious AP and mostly happened within 2 days to 1 month after starting therapy with some cases up to 1 year. Our case is unique due to the late onset of AP—after five years of therapy—well beyond the reported time frame. This case highlights the importance of ongoing clinical awareness of potential DIAP in long-term VDZ users and the need for further study of its association and risk factors, including possible effects of route of administration.
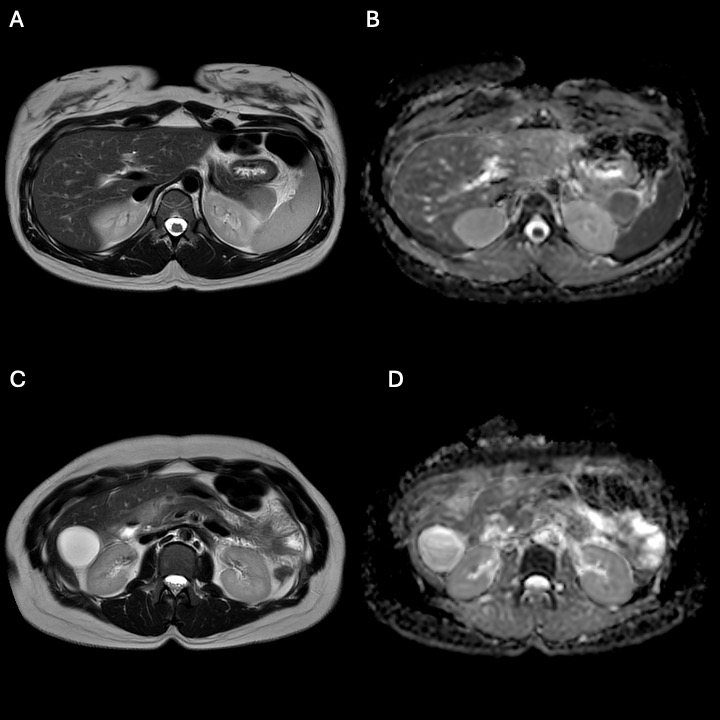
Figure: Table 1. Laboratory Data On Admission and Two Weeks After Discharge.
No end organ damage was noticed on admission along with normal inflammatory markers; however, her lipase and amylase were elevated over the course of 3 weeks.
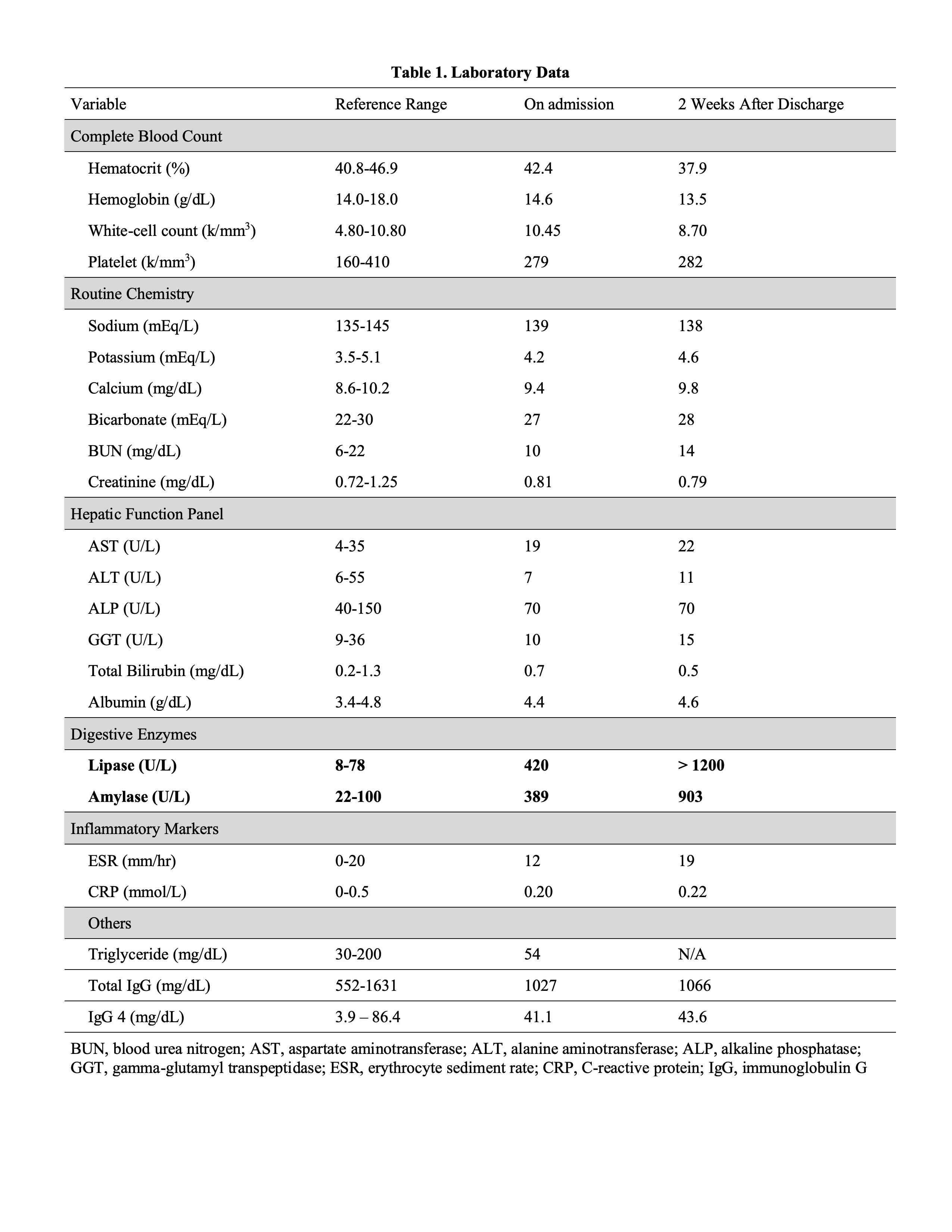
Figure: Figure 1. Magnetic Resonance Imaging (MRI) of Abdomen Showing Pancreatitis.
MRI of the abdomen showing pancreatic head (A, B) and tail (C, D) on T2 weighted imaging (A, C) and restrict diffusion (B, D) consistent with acute pancreatitis.
Disclosures:
Wei Tang indicated no relevant financial relationships.
Deepali Tewari indicated no relevant financial relationships.
Maxwell Charlat indicated no relevant financial relationships.
Virendra Tewari indicated no relevant financial relationships.
Wei Tang, MD1, Deepali Tewari, MD2, Maxwell Charlat, MD1, Virendra Tewari, MBBS, MD, DM, FACG2. P5549 - Vedolizumab-Associated Acute Pancreatitis After 5 Years of Use in a Patient With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Westchester Medical Center, Valhalla, NY; 2New York Medical College, Valhalla, NY
Introduction: Vedolizumab (VDZ), an α4β7 integrin inhibitor, is widely used in moderate-to-severe inflammatory bowel disease (IBD) due to its gut-selective action and favorable safety profile. Drug-induced acute pancreatitis (DIAP) is rare. We present a case of VDZ-associated acute pancreatitis (AP) in a patient with ulcerative colitis (UC) after 5 years of therapy.
Case Description/
Methods: A 32-year-old woman with left-sided UC (diagnosed 7 years ago, extending to the splenic flexure) and antiphospholipid syndrome (APLS) on aspirin for thromboprophylaxis presented with 4 days of severe, stabbing epigastric pain radiating to the back. She had ischemic colitis of the hepatic flexure 11 years ago and subsequent workup revealed APLS. She had been in clinical and endoscopic remission on VDZ infusions every 8 weeks for 5 years; her next dose was due in 4 days.
Initial labs (Table 1) showed elevated lipase (420 U/L) and amylase (389 U/L), with normal inflammatory markers and no signs of organ failure. She was hospitalized for 3 days for significant pain. Esophagogastroduodenoscopy (EGD) was unremarkable and upper endoscopic ultrasound (EUS) revealed normal appearing pancreas, common bile duct, and gallbladder. CT angiography showed normal vasculature. Magnetic resonance cholangiopancreatography (MRCP) revealed pancreatic head and tail edema, consistent with pancreatitis (Fig. 1). Further evaluation—including lipid panel, IgG4 level, and hereditary pancreatitis genetic panel—was unremarkable. With no other identifiable cause, DIAP was suspected.
Over 3-4 weeks her lipase and amylase were still elevated, but the inflammatory markers remained normal, and pain gradually improved. Her VDZ was delayed, and she opted to switch to subcutaneous formulation, which she tolerated without worsening pain.
Discussion: DIAP accounts for < 5% of AP and is a diagnosis of exclusion. A 2023 analysis of Takeda’s Global Safety Database ( >700,000 patient-years) reported only196 cases of pancreatitis in VDZ-treated IBD patients, almost all were serious AP and mostly happened within 2 days to 1 month after starting therapy with some cases up to 1 year. Our case is unique due to the late onset of AP—after five years of therapy—well beyond the reported time frame. This case highlights the importance of ongoing clinical awareness of potential DIAP in long-term VDZ users and the need for further study of its association and risk factors, including possible effects of route of administration.

Figure: Table 1. Laboratory Data On Admission and Two Weeks After Discharge.
No end organ damage was noticed on admission along with normal inflammatory markers; however, her lipase and amylase were elevated over the course of 3 weeks.

Figure: Figure 1. Magnetic Resonance Imaging (MRI) of Abdomen Showing Pancreatitis.
MRI of the abdomen showing pancreatic head (A, B) and tail (C, D) on T2 weighted imaging (A, C) and restrict diffusion (B, D) consistent with acute pancreatitis.
Disclosures:
Wei Tang indicated no relevant financial relationships.
Deepali Tewari indicated no relevant financial relationships.
Maxwell Charlat indicated no relevant financial relationships.
Virendra Tewari indicated no relevant financial relationships.
Wei Tang, MD1, Deepali Tewari, MD2, Maxwell Charlat, MD1, Virendra Tewari, MBBS, MD, DM, FACG2. P5549 - Vedolizumab-Associated Acute Pancreatitis After 5 Years of Use in a Patient With Ulcerative Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
