Tuesday Poster Session
Category: IBD
P5535 - Refractory Acute Severe UC Flare Responsive to Upadacitinib
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- NS
Naveena Sunkara, MD (she/her/hers)
Westchester Medical Center
Valhalla, NY
Presenting Author(s)
Naveena Sunkara, MD1, Moshe Spira, MD2, Dana Berg, MD1
1Westchester Medical Center, Valhalla, NY; 2Westchester Medical Center, Fair Lawn, NJ
Introduction: Acute severe ulcerative colitis (ASUC) requires prompt recognition and aggressive treatment to prevent poor outcomes. First line therapy includes IV steroids followed by Infliximab or cyclosporin if steroids fail. Janus kinase (JAK) 1 inhibitors such as Upadacitinib and Tofacitinib are approved for induction and maintenance of moderate to severe UC in outpatients but not as salvage therapy in ASUC. We present a case of ASUC in a hospitalized patient on an Infliximab biosimilar 5 mg/kg q8 weeks who was refractory to steroids but improved with Upadacitinib, highlighting the benefit of early use of JAK inhibitors in bio-exposed patients.
Case Description/
Methods: A 31-year-old man with UC (diagnosed 12 years prior) on an infliximab biosimilar (started nine months prior to presentation, previously on mesalamine) with prior clinical and endoscopic remission presented with three weeks of abdominal pain and 10 bloody bowel movements daily. Exam showed abdominal tenderness. Labs: WBC 12.64, Hgb 14.1, albumin 4.4 g/dL, calprotectin 5,570, CRP 4.70 mg/dL. Infectious work up was negative. Infliximab level was 0.8 mcg/mL with antibodies >100 AU. Abdominal x-ray was normal. GI was consulted; IV methylprednisolone 60 mg/daily was started. Sigmoidoscopy showed diffuse friability and ulceration (Mayo 3). Given active US on anti-TNF with subtherapeutic level and high antibodies, out-of-class therapy was pursued. JAK inhibitors were identified as a potential therapy given their rapid onset of action. Oral Upadacitinib 45 mg/day was started on hospital day 6. By day 8, he had no hematochezia, 1-2 formed stools daily and CRP normalized (0.39 mg/dL). He was discharged on day 11 with clinical improvement.
Discussion: Upadacitinib, a selective JAK-1 inhibitor, is approved for use for moderate-to-severe UC but not as salvage for ASUC. This case illustrates its successful off-label use as rescue therapy in a bio-exposed patient in the in-patient setting who developed immunogenicity to an anti-TNF agent as was unlikely to respond to dose escalation. Cyclosporin use is limited by the need for intensive monitoring and need to transition to other agents for outpatient care. Our patient had symptom resolution within 5 days of JAK inhibitor initiation. This supports considering Upadacitinib off-label for AUSC rescue in TNF-failure cases and highlights the need for randomized control trials to evaluate safety and efficacy in this setting.
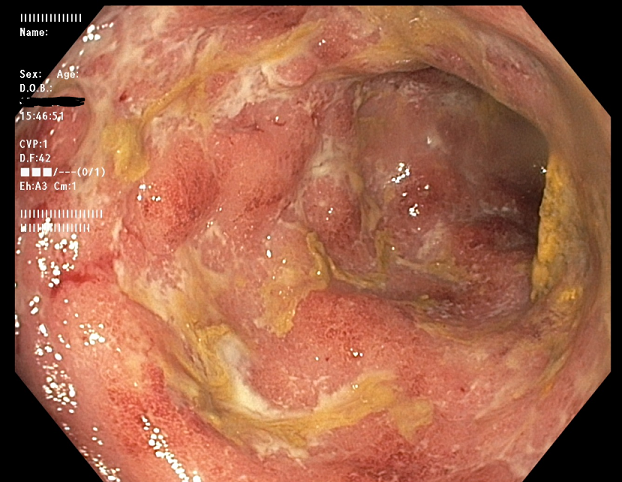
Figure: Sigmoid Ulceration
Disclosures:
Naveena Sunkara indicated no relevant financial relationships.
Moshe Spira indicated no relevant financial relationships.
Dana Berg indicated no relevant financial relationships.
Naveena Sunkara, MD1, Moshe Spira, MD2, Dana Berg, MD1. P5535 - Refractory Acute Severe UC Flare Responsive to Upadacitinib, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Westchester Medical Center, Valhalla, NY; 2Westchester Medical Center, Fair Lawn, NJ
Introduction: Acute severe ulcerative colitis (ASUC) requires prompt recognition and aggressive treatment to prevent poor outcomes. First line therapy includes IV steroids followed by Infliximab or cyclosporin if steroids fail. Janus kinase (JAK) 1 inhibitors such as Upadacitinib and Tofacitinib are approved for induction and maintenance of moderate to severe UC in outpatients but not as salvage therapy in ASUC. We present a case of ASUC in a hospitalized patient on an Infliximab biosimilar 5 mg/kg q8 weeks who was refractory to steroids but improved with Upadacitinib, highlighting the benefit of early use of JAK inhibitors in bio-exposed patients.
Case Description/
Methods: A 31-year-old man with UC (diagnosed 12 years prior) on an infliximab biosimilar (started nine months prior to presentation, previously on mesalamine) with prior clinical and endoscopic remission presented with three weeks of abdominal pain and 10 bloody bowel movements daily. Exam showed abdominal tenderness. Labs: WBC 12.64, Hgb 14.1, albumin 4.4 g/dL, calprotectin 5,570, CRP 4.70 mg/dL. Infectious work up was negative. Infliximab level was 0.8 mcg/mL with antibodies >100 AU. Abdominal x-ray was normal. GI was consulted; IV methylprednisolone 60 mg/daily was started. Sigmoidoscopy showed diffuse friability and ulceration (Mayo 3). Given active US on anti-TNF with subtherapeutic level and high antibodies, out-of-class therapy was pursued. JAK inhibitors were identified as a potential therapy given their rapid onset of action. Oral Upadacitinib 45 mg/day was started on hospital day 6. By day 8, he had no hematochezia, 1-2 formed stools daily and CRP normalized (0.39 mg/dL). He was discharged on day 11 with clinical improvement.
Discussion: Upadacitinib, a selective JAK-1 inhibitor, is approved for use for moderate-to-severe UC but not as salvage for ASUC. This case illustrates its successful off-label use as rescue therapy in a bio-exposed patient in the in-patient setting who developed immunogenicity to an anti-TNF agent as was unlikely to respond to dose escalation. Cyclosporin use is limited by the need for intensive monitoring and need to transition to other agents for outpatient care. Our patient had symptom resolution within 5 days of JAK inhibitor initiation. This supports considering Upadacitinib off-label for AUSC rescue in TNF-failure cases and highlights the need for randomized control trials to evaluate safety and efficacy in this setting.

Figure: Sigmoid Ulceration
Disclosures:
Naveena Sunkara indicated no relevant financial relationships.
Moshe Spira indicated no relevant financial relationships.
Dana Berg indicated no relevant financial relationships.
Naveena Sunkara, MD1, Moshe Spira, MD2, Dana Berg, MD1. P5535 - Refractory Acute Severe UC Flare Responsive to Upadacitinib, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

