Tuesday Poster Session
Category: IBD
P5529 - Risankizumab Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE): An Unexpected Skin Reaction in Ulcerative Colitis Treatment
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Charmy Parikh, MD
Mercy Catholic Medical Center
Darby, PA
Presenting Author(s)
Award: ACG Presidential Poster Award
Charmy Parikh, MD1, Raj H. Patel, MD2, Dhruvan Patel, MD1
1Mercy Catholic Medical Center, Darby, PA; 2St. Mary Medical Center, Langhorne, PA
Introduction: Ulcerative colitis (UC) is a chronic, immune-mediated inflammatory bowel disease and presents with diarrhea, rectal bleeding, and urgency. It has affected over 1.5 million people in North America. Risankizumab, which is an IL-23p19 inhibitor, has recently received FDA approval in June 2024 for moderate to severe UC, based on the 2 trials INSPIRE and COMMAND, which showcased its efficacy and safety in IBD. Here we present a case of a rare cutaneous condition associated with risankizumab use.
Case Description/
Methods: A 56-year-old male was diagnosed with UC in 2021 and was managed with mesalamine, he eventually experienced a UC flare with pancolitis in 2023. Following this, he was switched to infliximab, which led to clinical remission. During the course, he developed palmoplantar psoriasis, which was ascribed to anti-TNF therapy, and infliximab was discontinued.
He was started on risankizumab therapy in November 2024 and tolerated 3 induction infusions without any concerns. However, 3 days post-first subcutaneous maintenance dose in April 2025, he started developing a symmetrical erythematous rash more specific to flexural and intertriginous areas in addition to fungal infection in axillae. He received treatment with corticosteroids and antihistamines, and the rashes resolved. He received his second dose of risankizumab in May 2025, and the similar rashes recurred. He received a formal dermatological evaluation and was diagnosed with SDRIFE (Symmetrical Drug-Related Intertriginous and Flexural Exanthema) secondary to risankizumab use. We discontinued his risankizumab, and the patient received steroids and antihistamines as before. The patient was finally started on vedolizumab, and he currently remains in remission of UC both clinically and endoscopically.
Discussion: The above case reveals a rare but clinically significant adverse reaction of risankizumab in UC. SDRIFE (a delayed-type hypersensitivity reaction), also known as baboon syndrome, should be considered as a possible differential diagnosis for any flexural rashes occurring after IL-23 inhibitor use. Early identification and medication discontinuation are the keys to preventing recurrence and guiding safer alternatives for IBD management.
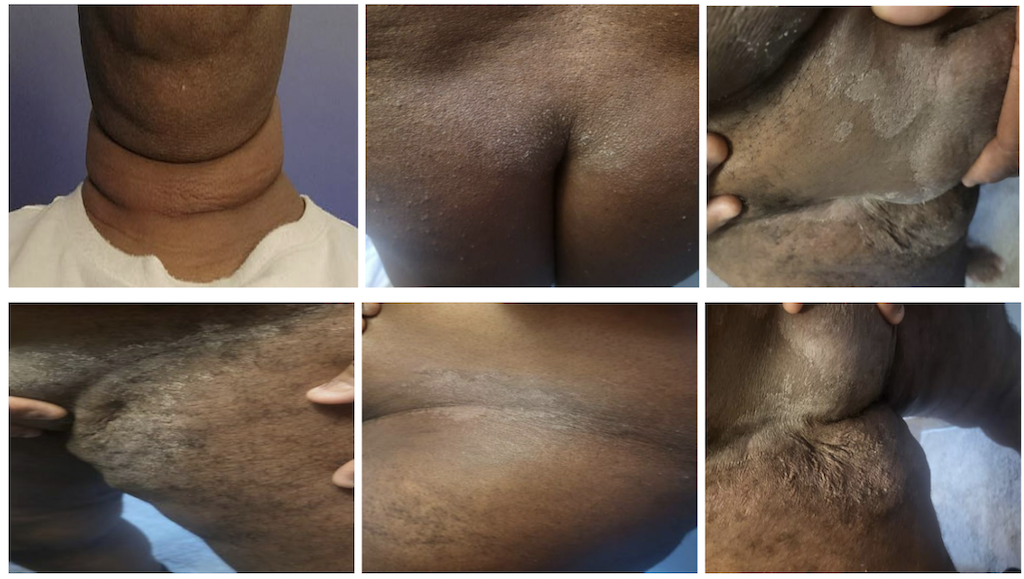
Figure: Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE) post risankizumab use
Disclosures:
Charmy Parikh indicated no relevant financial relationships.
Raj Patel indicated no relevant financial relationships.
Dhruvan Patel indicated no relevant financial relationships.
Charmy Parikh, MD1, Raj H. Patel, MD2, Dhruvan Patel, MD1. P5529 - Risankizumab Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE): An Unexpected Skin Reaction in Ulcerative Colitis Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Charmy Parikh, MD1, Raj H. Patel, MD2, Dhruvan Patel, MD1
1Mercy Catholic Medical Center, Darby, PA; 2St. Mary Medical Center, Langhorne, PA
Introduction: Ulcerative colitis (UC) is a chronic, immune-mediated inflammatory bowel disease and presents with diarrhea, rectal bleeding, and urgency. It has affected over 1.5 million people in North America. Risankizumab, which is an IL-23p19 inhibitor, has recently received FDA approval in June 2024 for moderate to severe UC, based on the 2 trials INSPIRE and COMMAND, which showcased its efficacy and safety in IBD. Here we present a case of a rare cutaneous condition associated with risankizumab use.
Case Description/
Methods: A 56-year-old male was diagnosed with UC in 2021 and was managed with mesalamine, he eventually experienced a UC flare with pancolitis in 2023. Following this, he was switched to infliximab, which led to clinical remission. During the course, he developed palmoplantar psoriasis, which was ascribed to anti-TNF therapy, and infliximab was discontinued.
He was started on risankizumab therapy in November 2024 and tolerated 3 induction infusions without any concerns. However, 3 days post-first subcutaneous maintenance dose in April 2025, he started developing a symmetrical erythematous rash more specific to flexural and intertriginous areas in addition to fungal infection in axillae. He received treatment with corticosteroids and antihistamines, and the rashes resolved. He received his second dose of risankizumab in May 2025, and the similar rashes recurred. He received a formal dermatological evaluation and was diagnosed with SDRIFE (Symmetrical Drug-Related Intertriginous and Flexural Exanthema) secondary to risankizumab use. We discontinued his risankizumab, and the patient received steroids and antihistamines as before. The patient was finally started on vedolizumab, and he currently remains in remission of UC both clinically and endoscopically.
Discussion: The above case reveals a rare but clinically significant adverse reaction of risankizumab in UC. SDRIFE (a delayed-type hypersensitivity reaction), also known as baboon syndrome, should be considered as a possible differential diagnosis for any flexural rashes occurring after IL-23 inhibitor use. Early identification and medication discontinuation are the keys to preventing recurrence and guiding safer alternatives for IBD management.

Figure: Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE) post risankizumab use
Disclosures:
Charmy Parikh indicated no relevant financial relationships.
Raj Patel indicated no relevant financial relationships.
Dhruvan Patel indicated no relevant financial relationships.
Charmy Parikh, MD1, Raj H. Patel, MD2, Dhruvan Patel, MD1. P5529 - Risankizumab Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema (SDRIFE): An Unexpected Skin Reaction in Ulcerative Colitis Treatment, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

