Tuesday Poster Session
Category: IBD
P5520 - JAK of All Trades: Upadacitinib-Induced Remission of Medically Refractory Lymphocytic Colitis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Kevin R. O'Connor, MD
California Pacific Medical Center
San Francisco, CA
Presenting Author(s)
Kevin R. O'Connor, MD1, Ryan A. McConnell, MD2, Anna Thiemann, MD1
1California Pacific Medical Center, San Francisco, CA; 2Palo Alto Medical Foundation, Sutter Health, Palo Alto, CA
Introduction: Microscopic colitis (MC) is an idiopathic, inflammatory colitis that causes chronic watery diarrhea and adversely impacts quality of life. Remission is defined clinically with the Hjortswang criteria (mean of < 3 stools/day and < 1 watery stool/day). Most patients respond to budesonide therapy, although relapse rates are high. Approximately 20% of patients have steroid-refractory disease. Guidelines recommend thiopurines, anti-TNF agents, and vedolizumab for budesonide-refractory MC. There is a paucity of data supporting these off-label advanced therapies, particularly for newer agents, such as the JAK inhibitors. Here, we present a case of upadacitinib-induced clinical remission of steroid-refractory lymphocytic colitis (LC) in a patient with concurrent celiac disease.
Case Description/
Methods: A 38-year-old female with well-controlled celiac disease and LC previously responsive to budesonide presented with recurrent loose stools (4 stools/day), urgency, and incontinence. Colonoscopy confirmed active LC. The patient was not taking medications associated with MC risk. Stool pathogens were excluded, and tissue transglutaminase IgA suggested good celiac disease control. An 8-week course of ileal-release budesonide 9 mg daily did not induce remission. Off-label vedolizumab and adalimumab failed to induce symptomatic remission, and repeat histologic evaluations confirmed persistent LC activity. She began upadacitinib 45 mg daily induction dosing. Within one week, she achieved clinical remission with 1-2 Bristol Type 4 stools daily and no urgency or incontinence. After an 8-week induction, she transitioned to upadacitinib 30 mg daily maintenance dosing. Symptomatic remission has been sustained for over 11 months with no serious adverse effects.
Discussion: Budesonide-refractory MC has a significant adverse impact on quality of life and no FDA-approved therapies. Case series show the potential efficacy of off-label advanced inflammatory bowel disease therapies (primarily anti-TNF and vedolizumab) in this setting. We’ve identified published cases of JAK inhibitor therapy for refractory MC, describing upadacitinib in six cases and tofacitinib in one case. In all cases, including ours, clinical remission was achieved rapidly (within days). Our patient’s rapid and sustained improvement with upadacitinib adds to the emerging evidence supporting the potential role for JAK inhibition as a refractory MC therapy. Future exploration in controlled studies is warranted.
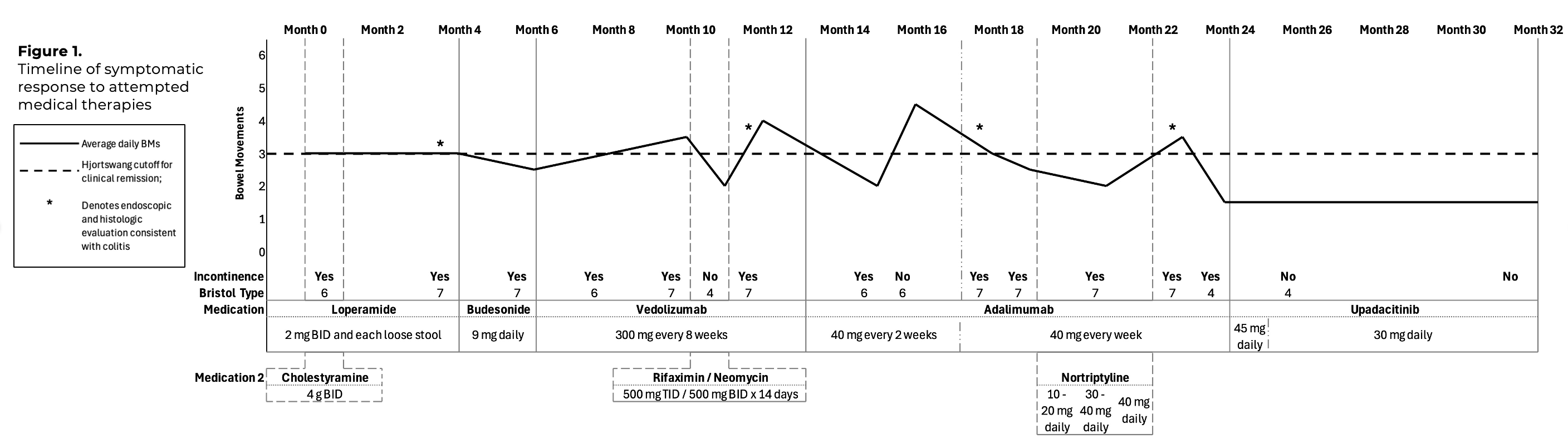
Figure: Timeline of symptomatic response to attempted medical therapies.
Disclosures:
Kevin O'Connor indicated no relevant financial relationships.
Ryan McConnell: AbbVie – Speakers Bureau. Eli Lilly – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Pfizer – Speakers Bureau. Takeda – Speakers Bureau.
Anna Thiemann indicated no relevant financial relationships.
Kevin R. O'Connor, MD1, Ryan A. McConnell, MD2, Anna Thiemann, MD1. P5520 - JAK of All Trades: Upadacitinib-Induced Remission of Medically Refractory Lymphocytic Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1California Pacific Medical Center, San Francisco, CA; 2Palo Alto Medical Foundation, Sutter Health, Palo Alto, CA
Introduction: Microscopic colitis (MC) is an idiopathic, inflammatory colitis that causes chronic watery diarrhea and adversely impacts quality of life. Remission is defined clinically with the Hjortswang criteria (mean of < 3 stools/day and < 1 watery stool/day). Most patients respond to budesonide therapy, although relapse rates are high. Approximately 20% of patients have steroid-refractory disease. Guidelines recommend thiopurines, anti-TNF agents, and vedolizumab for budesonide-refractory MC. There is a paucity of data supporting these off-label advanced therapies, particularly for newer agents, such as the JAK inhibitors. Here, we present a case of upadacitinib-induced clinical remission of steroid-refractory lymphocytic colitis (LC) in a patient with concurrent celiac disease.
Case Description/
Methods: A 38-year-old female with well-controlled celiac disease and LC previously responsive to budesonide presented with recurrent loose stools (4 stools/day), urgency, and incontinence. Colonoscopy confirmed active LC. The patient was not taking medications associated with MC risk. Stool pathogens were excluded, and tissue transglutaminase IgA suggested good celiac disease control. An 8-week course of ileal-release budesonide 9 mg daily did not induce remission. Off-label vedolizumab and adalimumab failed to induce symptomatic remission, and repeat histologic evaluations confirmed persistent LC activity. She began upadacitinib 45 mg daily induction dosing. Within one week, she achieved clinical remission with 1-2 Bristol Type 4 stools daily and no urgency or incontinence. After an 8-week induction, she transitioned to upadacitinib 30 mg daily maintenance dosing. Symptomatic remission has been sustained for over 11 months with no serious adverse effects.
Discussion: Budesonide-refractory MC has a significant adverse impact on quality of life and no FDA-approved therapies. Case series show the potential efficacy of off-label advanced inflammatory bowel disease therapies (primarily anti-TNF and vedolizumab) in this setting. We’ve identified published cases of JAK inhibitor therapy for refractory MC, describing upadacitinib in six cases and tofacitinib in one case. In all cases, including ours, clinical remission was achieved rapidly (within days). Our patient’s rapid and sustained improvement with upadacitinib adds to the emerging evidence supporting the potential role for JAK inhibition as a refractory MC therapy. Future exploration in controlled studies is warranted.

Figure: Timeline of symptomatic response to attempted medical therapies.
Disclosures:
Kevin O'Connor indicated no relevant financial relationships.
Ryan McConnell: AbbVie – Speakers Bureau. Eli Lilly – Speakers Bureau. Johnson & Johnson – Speakers Bureau. Pfizer – Speakers Bureau. Takeda – Speakers Bureau.
Anna Thiemann indicated no relevant financial relationships.
Kevin R. O'Connor, MD1, Ryan A. McConnell, MD2, Anna Thiemann, MD1. P5520 - JAK of All Trades: Upadacitinib-Induced Remission of Medically Refractory Lymphocytic Colitis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

