Tuesday Poster Session
Category: IBD
P5478 - Incremental Improvement in Bowel Urgency Was Associated With Improved Patient-Reported Outcomes: Post Hoc Results From LUCENT-1 and -2 Trials
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- JW
Jianmin Wu
Eli Lilly and Company
Indianapolis, IN
Presenting Author(s)
Marla C. Dubinsky, MD1, Simon Travis, 2, Jianmin Wu, 3, Baojin Zhu, 3, Hanbo Qiu, 3, Chasity Mosby, 3, Sarah Folian, 3, Taku Kobayashi, 4, Badr Al Bawardy, MD5, David T. Rubin, MD6
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom; 3Eli Lilly and Company, Indianapolis, IN; 4Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Tokyo, Japan; 5Yale New Haven Hospital, New Haven, USA; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, New Haven, CT; 6University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Bowel urgency (BU) is a bothersome symptom experienced by patients with ulcerative colitis (UC) and is associated with high clinical and quality-of-life burdens. Mirikizumab (MIRI), an anti-IL-23 antibody approved for the treatment of moderately-to-severely active UC, significantly reduced BU severity compared with placebo (PBO), and these improvements were associated with better clinical outcomes. We evaluated the association between incremental improvement in BU severity at Week (W)12 and W52 with other patient-reported outcomes for MIRI-treated patients.
Methods: In LUCENT-1 (induction), patients were randomized 3:1 to intravenous MIRI 300 mg or PBO every 4 weeks (Q4W) for 12W. MIRI responders during induction were re-randomized 2:1 to subcutaneous MIRI 200 mg or PBO Q4W for 40W in LUCENT-2 (52W of continuous treatment). BU severity was measured using the Urgency Numeric Rating Scale ([UNRS] 0–10). Incremental improvement of BU severity was assessed as reduction of each single point of UNRS score from baseline (BL). The reduction of >7points was pooled into one category due to sample size. The accumulative benefit of reducing 1 additional point of UNRS score was evaluated at W12 and W52 and was assessed for its association with mean percent improvement in inflammatory bowel disease questionnaire (IBDQ) total score, fatigue NRS, short-form (SF)-36 mental component score (MCS) and physical component score (PCS), and work productivity activity impairment (WPAI) productivity loss.
Results: Significantly greater proportions of patients receiving MIRI achieved UNRS score reduction of ≥1 point through ≥7 points versus PBO at W12 and W52. The reduction of each single point of UNRS score was associated with improvement in patient’s physical and mental health, fatigue, work productivity, and quality of life. At W12, fatigue and IBDQ showed greater percent improvements among all the PRO outcomes studied for each 1-point reduction of UNRS. Similar results were observed at W52 (Table).
Discussion: Incremental improvement in bowel urgency corresponded to improvements in outcomes important to patients, underscoring the importance of managing BU symptoms in improving the quality of life for patients with UC.
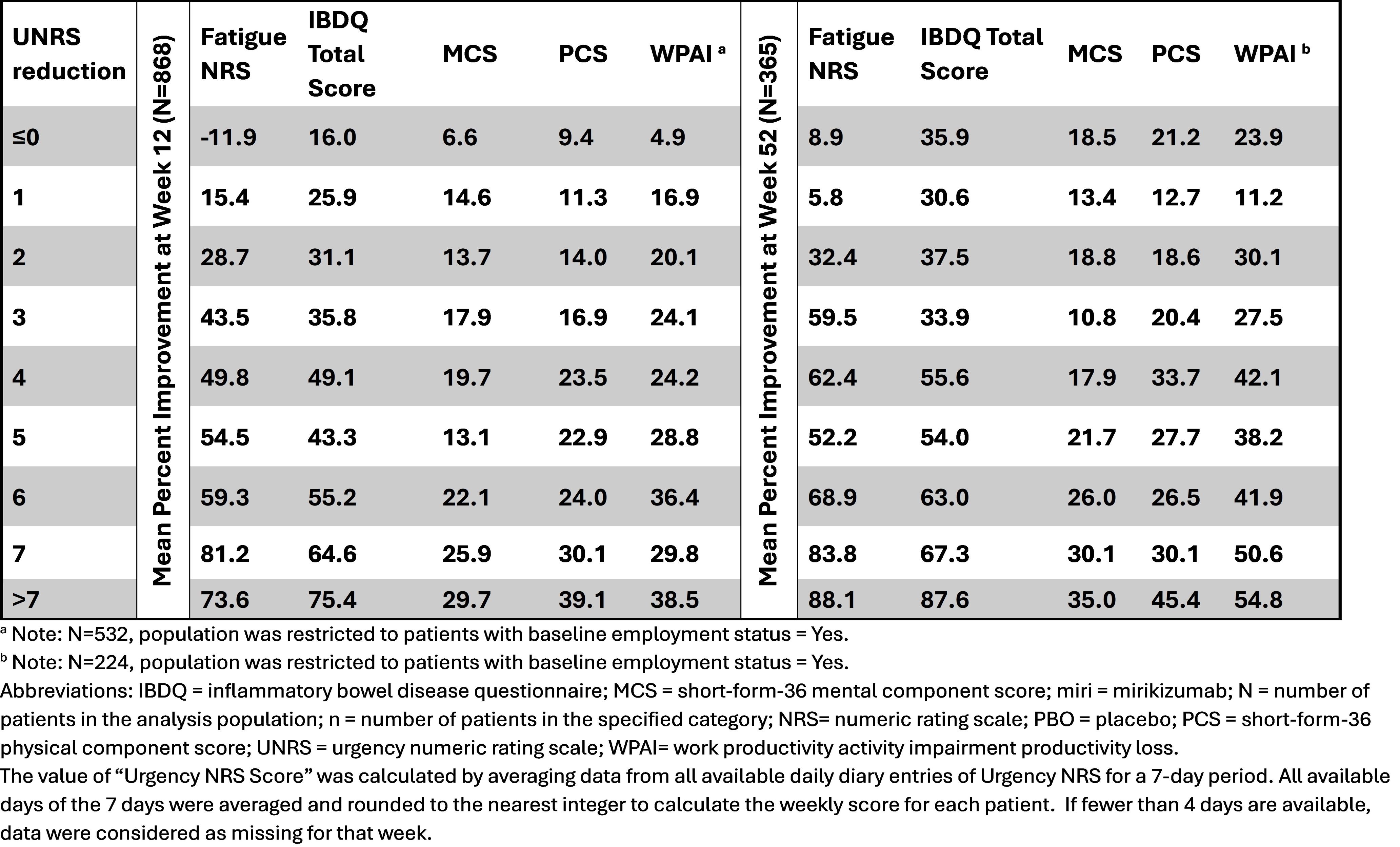
Figure: Mean Percent Improvement in Patient Reported Outcomes at Weeks 12 And 52 by UNRS Reduction in Patients Receiving Mirikizumab
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Simon Travis: AbbVie – Grant/Research Support, Honoraria, Travel expenses. Alimentiv – Independent Contractor. Bioclinica – Consultant. Bristol Myers Squibb – Consultant. Cosmo – Consultant. Dova Health Intelligence – Consultant, Stock Options. ECCO Health Care – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support, Honoraria. Equillium – Consultant. Ferring – Consultant, Honoraria, Travel expenses. Genentech – Consultant. GlaxoSmithKline – Consultant. Immunocore – Consultant. Immunometabolism – Consultant. Janssen – Consultant. Mestag – Consultant. MSD – Consultant. Norman Collisson Foundation – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support, Travel expenses. Protagonist – Consultant. Roche – Consultant. Satisfai – Consultant. Sorriso – Consultant. Spyre – Consultant. Takeda – Consultant, Honoraria, Travel expenses. The Helmsley Trust – Grant/Research Support. the International Organization for the Study of Inflammatory Bowel Diseases – Grant/Research Support. UCB – Consultant, Grant/Research Support. UK–India Education and Research Initiative – Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Baojin Zhu: Eli Lilly and Company – Employee, Stock Options.
Hanbo Qiu: Eli Lilly and Company – Employee, Stock Options.
Chasity Mosby: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ajinomoto Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Medical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eisai – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Thermo Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Badr Al Bawardy: AbbVie – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Hikma – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. TAKEDA – Advisor or Review Panel Member, Speakers Bureau.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Marla C. Dubinsky, MD1, Simon Travis, 2, Jianmin Wu, 3, Baojin Zhu, 3, Hanbo Qiu, 3, Chasity Mosby, 3, Sarah Folian, 3, Taku Kobayashi, 4, Badr Al Bawardy, MD5, David T. Rubin, MD6. P5478 - Incremental Improvement in Bowel Urgency Was Associated With Improved Patient-Reported Outcomes: Post Hoc Results From LUCENT-1 and -2 Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Translational Gastroenterology Unit, University of Oxford and Oxford Biomedical Research Centre, Oxford, UK, Oxford, England, United Kingdom; 3Eli Lilly and Company, Indianapolis, IN; 4Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Tokyo, Japan; 5Yale New Haven Hospital, New Haven, USA; King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia, New Haven, CT; 6University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Bowel urgency (BU) is a bothersome symptom experienced by patients with ulcerative colitis (UC) and is associated with high clinical and quality-of-life burdens. Mirikizumab (MIRI), an anti-IL-23 antibody approved for the treatment of moderately-to-severely active UC, significantly reduced BU severity compared with placebo (PBO), and these improvements were associated with better clinical outcomes. We evaluated the association between incremental improvement in BU severity at Week (W)12 and W52 with other patient-reported outcomes for MIRI-treated patients.
Methods: In LUCENT-1 (induction), patients were randomized 3:1 to intravenous MIRI 300 mg or PBO every 4 weeks (Q4W) for 12W. MIRI responders during induction were re-randomized 2:1 to subcutaneous MIRI 200 mg or PBO Q4W for 40W in LUCENT-2 (52W of continuous treatment). BU severity was measured using the Urgency Numeric Rating Scale ([UNRS] 0–10). Incremental improvement of BU severity was assessed as reduction of each single point of UNRS score from baseline (BL). The reduction of >7points was pooled into one category due to sample size. The accumulative benefit of reducing 1 additional point of UNRS score was evaluated at W12 and W52 and was assessed for its association with mean percent improvement in inflammatory bowel disease questionnaire (IBDQ) total score, fatigue NRS, short-form (SF)-36 mental component score (MCS) and physical component score (PCS), and work productivity activity impairment (WPAI) productivity loss.
Results: Significantly greater proportions of patients receiving MIRI achieved UNRS score reduction of ≥1 point through ≥7 points versus PBO at W12 and W52. The reduction of each single point of UNRS score was associated with improvement in patient’s physical and mental health, fatigue, work productivity, and quality of life. At W12, fatigue and IBDQ showed greater percent improvements among all the PRO outcomes studied for each 1-point reduction of UNRS. Similar results were observed at W52 (Table).
Discussion: Incremental improvement in bowel urgency corresponded to improvements in outcomes important to patients, underscoring the importance of managing BU symptoms in improving the quality of life for patients with UC.

Figure: Mean Percent Improvement in Patient Reported Outcomes at Weeks 12 And 52 by UNRS Reduction in Patients Receiving Mirikizumab
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
Simon Travis: AbbVie – Grant/Research Support, Honoraria, Travel expenses. Alimentiv – Independent Contractor. Bioclinica – Consultant. Bristol Myers Squibb – Consultant. Cosmo – Consultant. Dova Health Intelligence – Consultant, Stock Options. ECCO Health Care – Grant/Research Support. Eli Lilly and Company – Consultant, Grant/Research Support, Honoraria. Equillium – Consultant. Ferring – Consultant, Honoraria, Travel expenses. Genentech – Consultant. GlaxoSmithKline – Consultant. Immunocore – Consultant. Immunometabolism – Consultant. Janssen – Consultant. Mestag – Consultant. MSD – Consultant. Norman Collisson Foundation – Grant/Research Support. Novartis – Consultant. Pfizer – Consultant, Grant/Research Support, Travel expenses. Protagonist – Consultant. Roche – Consultant. Satisfai – Consultant. Sorriso – Consultant. Spyre – Consultant. Takeda – Consultant, Honoraria, Travel expenses. The Helmsley Trust – Grant/Research Support. the International Organization for the Study of Inflammatory Bowel Diseases – Grant/Research Support. UCB – Consultant, Grant/Research Support. UK–India Education and Research Initiative – Grant/Research Support. Vifor – Consultant, Grant/Research Support.
Jianmin Wu: Eli Lilly and Company – Employee, Stock Options.
Baojin Zhu: Eli Lilly and Company – Employee, Stock Options.
Hanbo Qiu: Eli Lilly and Company – Employee, Stock Options.
Chasity Mosby: Eli Lilly and Company – Employee, Stock Options.
Sarah Folian: Eli Lilly and Company – Employee, Stock Options.
Taku Kobayashi: AbbVie – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Ajinomoto Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Alfresa Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Asahi Kasei Medical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Astellas – Advisory Committee/Board Member, Consultant, Speakers Bureau. Celltrion – Advisory Committee/Board Member, Consultant, Speakers Bureau. Covidien – Advisory Committee/Board Member, Consultant, Speakers Bureau. EA Pharma – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Eisai – Advisory Committee/Board Member, Consultant, Speakers Bureau. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speakers Bureau. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speakers Bureau. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speakers Bureau. Janssen – Advisory Committee/Board Member, Consultant, Speakers Bureau. JIMRO – Advisory Committee/Board Member, Consultant, Speakers Bureau. Kyorin Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Mitsubishi Tanabe Pharma – Advisory Committee/Board Member, Consultant, Speakers Bureau. Mochida Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Nippon Kayaku – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Otsuka Holdings – Grant/Research Support. Pfizer – Advisory Committee/Board Member, Consultant, Speakers Bureau. Sekisui Medical – Grant/Research Support. Takeda Pharmaceutical – Advisory Committee/Board Member, Consultant, Speakers Bureau. Thermo Scientific – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau. Zeria Pharmaceutical – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speakers Bureau.
Badr Al Bawardy: AbbVie – Advisor or Review Panel Member, Consultant, Speakers Bureau. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Hikma – Speakers Bureau. Janssen – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member. TAKEDA – Advisor or Review Panel Member, Speakers Bureau.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Marla C. Dubinsky, MD1, Simon Travis, 2, Jianmin Wu, 3, Baojin Zhu, 3, Hanbo Qiu, 3, Chasity Mosby, 3, Sarah Folian, 3, Taku Kobayashi, 4, Badr Al Bawardy, MD5, David T. Rubin, MD6. P5478 - Incremental Improvement in Bowel Urgency Was Associated With Improved Patient-Reported Outcomes: Post Hoc Results From LUCENT-1 and -2 Trials, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
