Tuesday Poster Session
Category: IBD
P5469 - Trends in the Use and Costs of Inflammatory Bowel Disease Advanced Therapies in the US, 2013-2022
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- PN
Pranay Nadella, MD, MPhil (he/him/his)
Brigham and Women's Hospital
Boston, MA
Presenting Author(s)
Pranay Nadella, MD, MPhil1, Mike Zhai, MD, MBA1, Jessica R.. Allegretti, MD, MPH2, Benjamin Rome, MD3
1Brigham and Women's Hospital, Boston, MA; 2Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Harvard Medical School, Boston, MA
Introduction: Over the past two decades, advanced therapies have revolutionized treatment for IBD. Newer drugs are often more expensive than older treatments, raising concerns about affordability to patients and the health care system. This study evaluates trends in the use and cost of advanced therapies as first-line treatments for IBD.
Methods: We conducted a retrospective cohort study of commercially insured IBD patients from a national claims database (Merative MarketScan) who initiated one of 11 advanced therapies from 2013 to 2022 and had no prior advanced therapy use. We measured trends in the proportion of patients initiating each therapy and estimated net annual treatment costs; for clinician-administered drugs (e.g. infliximab), costs were based on CMS Average Sales Prices, and for pharmacy-administered drugs, costs were estimated as wholesale acquisition costs (i.e., list prices) minus average manufacturer rebates from SSR health. All costs were reported in 2022 dollars. We used logistic regression to assess demographic and clinical characteristics associated with first-line use of ustekinumab and vedolizumab versus TNF-alpha inhibitors.
Results: Among 54,269 new users of IBD advanced therapies, the average age was 38 years (SD 16), 51% were female and fewer than 9% had other autoimmune diseases. 51% had Crohn’s Disease, 34% had UC, and 16% were unclassified (Table 1A). In 2013, nearly all patients started TNF-alpha inhibitors (infliximab, adalimumab); in 2022, 34% used adalimumab, 27% infliximab, and 38% used newer therapies, including vedolizumab (19%) and ustekinumab (15%) (Figure 1A). Compared to Crohn’s patients, those with UC were less likely (OR: 0.35, 95% CI: 0.31-0.39) to start ustekinumab and more likely to start vedolizumab (OR: 2.15, 95% CI: 2.01-2.30), both compared to starting TNF-alpha inhibitors (Tables 1B-C). From 2013 to 2022, the mean net average annual cost for first-line IBD advanced therapies increased by 36%, from $33,699 to $45,703 (Figure 1B).
Discussion: Costs for advanced therapies rose by 36% from 2013 to 2022, driven by price increases for some TNF-alpha inhibitors and greater use of new, more expensive agents. Understanding these trends can help clinicians engage in value-conscious prescribing, especially when therapeutic alternatives exist. While patient assistance programs reduce out-of-pocket costs for some patients, the burden to the healthcare system remains substantial—highlighting the need for more cost-transparent, evidence-aligned treatment strategies.
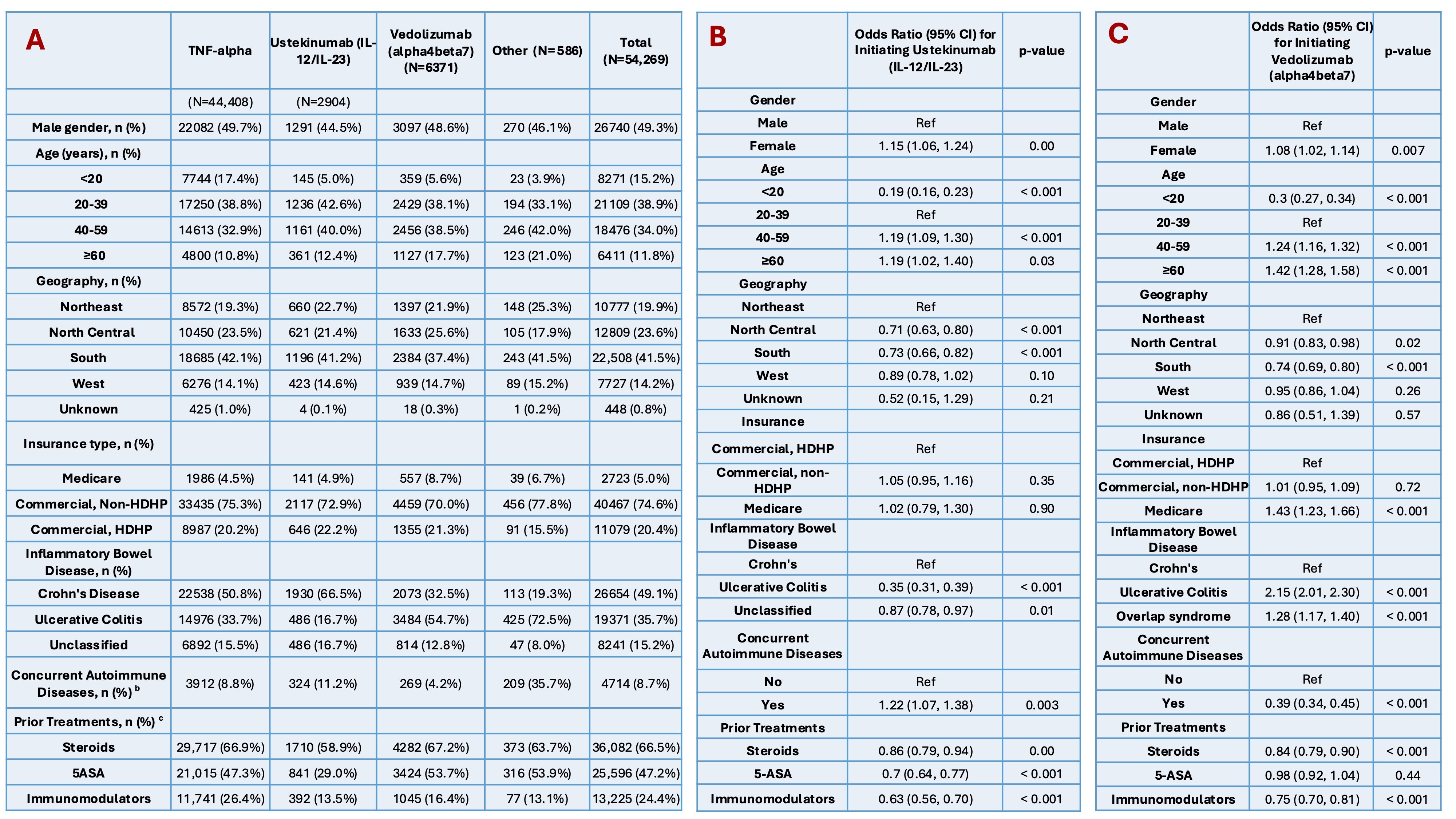
Figure: Table 1. A) Patient Characteristics of First-Line Biologic Treatments for Inflammatory Bowel Disease: TNF-alpha inhibitors vs Ustekinumab (IL-12/IL-23) vs Vedolizumab (alpha4beta7) vs Other. B) Factors associated with initiating Ustekinumab (Il-12/IL-23) vs TNF-alpha inhibitors. C) Factors associated with initiating Vedolizumab (alpha4beta7) vs TNF-alpha inhibitors.
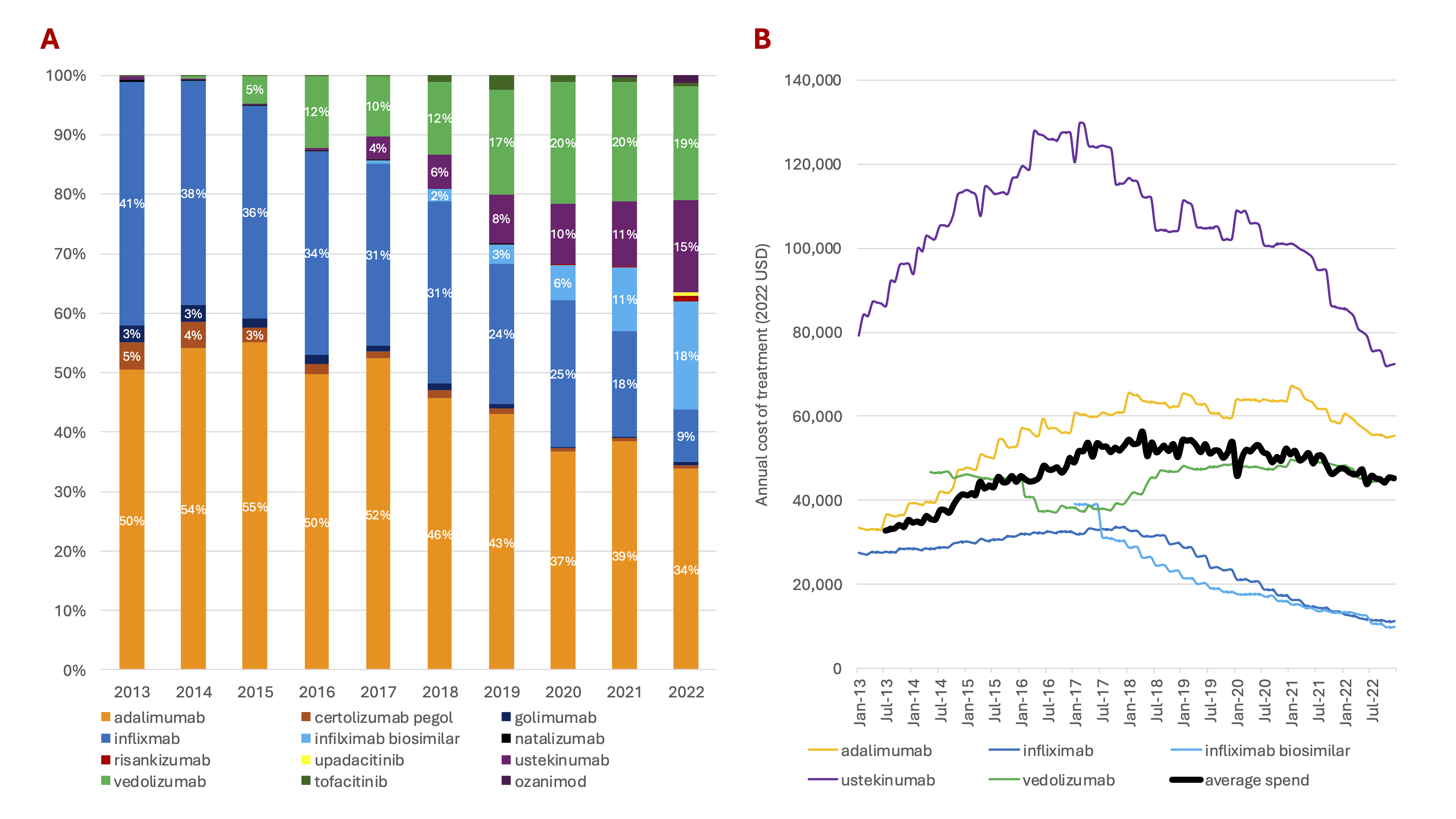
Figure: Figure 1. A) Relative Use of Advanced Therapies (2013-2022). B) Annual Average Net Cost of Treatment for Select Advanced Therapies (2013-2022).
Disclosures:
Pranay Nadella indicated no relevant financial relationships.
Mike Zhai indicated no relevant financial relationships.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Benjamin Rome indicated no relevant financial relationships.
Pranay Nadella, MD, MPhil1, Mike Zhai, MD, MBA1, Jessica R.. Allegretti, MD, MPH2, Benjamin Rome, MD3. P5469 - Trends in the Use and Costs of Inflammatory Bowel Disease Advanced Therapies in the US, 2013-2022, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Brigham and Women's Hospital, Boston, MA; 2Division of Gastroenterology, Hepatology, and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; 3Harvard Medical School, Boston, MA
Introduction: Over the past two decades, advanced therapies have revolutionized treatment for IBD. Newer drugs are often more expensive than older treatments, raising concerns about affordability to patients and the health care system. This study evaluates trends in the use and cost of advanced therapies as first-line treatments for IBD.
Methods: We conducted a retrospective cohort study of commercially insured IBD patients from a national claims database (Merative MarketScan) who initiated one of 11 advanced therapies from 2013 to 2022 and had no prior advanced therapy use. We measured trends in the proportion of patients initiating each therapy and estimated net annual treatment costs; for clinician-administered drugs (e.g. infliximab), costs were based on CMS Average Sales Prices, and for pharmacy-administered drugs, costs were estimated as wholesale acquisition costs (i.e., list prices) minus average manufacturer rebates from SSR health. All costs were reported in 2022 dollars. We used logistic regression to assess demographic and clinical characteristics associated with first-line use of ustekinumab and vedolizumab versus TNF-alpha inhibitors.
Results: Among 54,269 new users of IBD advanced therapies, the average age was 38 years (SD 16), 51% were female and fewer than 9% had other autoimmune diseases. 51% had Crohn’s Disease, 34% had UC, and 16% were unclassified (Table 1A). In 2013, nearly all patients started TNF-alpha inhibitors (infliximab, adalimumab); in 2022, 34% used adalimumab, 27% infliximab, and 38% used newer therapies, including vedolizumab (19%) and ustekinumab (15%) (Figure 1A). Compared to Crohn’s patients, those with UC were less likely (OR: 0.35, 95% CI: 0.31-0.39) to start ustekinumab and more likely to start vedolizumab (OR: 2.15, 95% CI: 2.01-2.30), both compared to starting TNF-alpha inhibitors (Tables 1B-C). From 2013 to 2022, the mean net average annual cost for first-line IBD advanced therapies increased by 36%, from $33,699 to $45,703 (Figure 1B).
Discussion: Costs for advanced therapies rose by 36% from 2013 to 2022, driven by price increases for some TNF-alpha inhibitors and greater use of new, more expensive agents. Understanding these trends can help clinicians engage in value-conscious prescribing, especially when therapeutic alternatives exist. While patient assistance programs reduce out-of-pocket costs for some patients, the burden to the healthcare system remains substantial—highlighting the need for more cost-transparent, evidence-aligned treatment strategies.

Figure: Table 1. A) Patient Characteristics of First-Line Biologic Treatments for Inflammatory Bowel Disease: TNF-alpha inhibitors vs Ustekinumab (IL-12/IL-23) vs Vedolizumab (alpha4beta7) vs Other. B) Factors associated with initiating Ustekinumab (Il-12/IL-23) vs TNF-alpha inhibitors. C) Factors associated with initiating Vedolizumab (alpha4beta7) vs TNF-alpha inhibitors.

Figure: Figure 1. A) Relative Use of Advanced Therapies (2013-2022). B) Annual Average Net Cost of Treatment for Select Advanced Therapies (2013-2022).
Disclosures:
Pranay Nadella indicated no relevant financial relationships.
Mike Zhai indicated no relevant financial relationships.
Jessica Allegretti: Abbvie – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker, Speakers Bureau. Adiso – Consultant. Bristol Myer Squibb – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speaker. Celltrion – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Ferring – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Finch – Consultant. Genentech – Advisory Committee/Board Member, Consultant. GlaxoSmithKline – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Iterative Scopes – Consultant. Janssen – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Speakers Bureau. Johnson & Johnson – Consultant, Grant/Research Support, Speaker. Merck – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Pfizer – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant, Grant/Research Support. Roivant – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Roivant Adiso – Consultant. Seres Therapeutics – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Shattuck Labs – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. TRXBio – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant. Vedanta – Advisor or Review Panel Member, Advisory Committee/Board Member, Consultant.
Benjamin Rome indicated no relevant financial relationships.
Pranay Nadella, MD, MPhil1, Mike Zhai, MD, MBA1, Jessica R.. Allegretti, MD, MPH2, Benjamin Rome, MD3. P5469 - Trends in the Use and Costs of Inflammatory Bowel Disease Advanced Therapies in the US, 2013-2022, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

