Tuesday Poster Session
Category: IBD
P5462 - Shorter Disease Duration Increases Clinical Remission Rates in Real-World Observational Studies for Crohn’s Disease, but Not Ulcerative Colitis: A Systematic Review and Meta-Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Michael Scott Dunn, MD
UNC School of Medicine
Chapel Hill, NC
Presenting Author(s)
Michael Scott. Dunn, MD1, John P.. Haydek, MD, MS2, Edward L. Barnes, MD, MPH3
1UNC School of Medicine, Chapel Hill, NC; 2Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, of North Carolina at Chapel Hill, Chapel Hill, NC; 3Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Although previous clinical trials and observational studies have shown a correlation between early intervention with advanced therapies and improved clinical outcomes in inflammatory bowel disease, no comprehensive review has been performed. The goal of this review was to evaluate the effect of disease duration on clinical remission rates in real-world studies.
Methods: We searched Cochrane, Embase, PubMed, and Scopus for retrospective and prospective cohort studies on adult patients (>18 years) with Crohn’s Disease (CD) or Ulcerative Colitis (UC) treated with tumor necrosis factor (TNF) antagonists, anti-integrin, anti-interleukin (IL)-12 and IL-23 agents, Janus Kinase (JAK) inhibitors, or Sphingosine-1-Phosphate (S1P) receptor modulators. Data was extracted for mean disease duration at time of therapy initiation, clinical remission, and clinical response rates at the end of induction (week 12-16) and maintenance (week 52-56) phases of treatment. A meta-analysis with single-factor analysis of variation (ANOVA) was performed to analyze the correlation between disease duration, year of study publication, and clinical remission and response rates.
Results: The search strategy yielded 5,947 citations, of which 141 studies (83 UC, 64 CD) were eligible for inclusion. Among studies evaluating CD, participants with a shorter mean disease duration (< 8 years) compared to longer disease duration (> 8 years), achieved higher rates of clinical remission at the end of induction phase (60.7% vs 40.6%) and maintenance phase (66.1% vs 48.8%) (P < 0.05 for all comparisons, Table 1). In contrast, mean disease duration was not significantly associated with clinical remission in studies evaluating UC. In an exploratory analysis among patients with CD, there was no difference in clinical remission for induction phase or maintenance phase when comparing participants with a disease duration <5 years to those with a duration of 5-8 years (53.0% vs 62.6%) and (64.1% vs 68.1%) respectively.
Discussion: In this systematic review and meta-analysis, we demonstrated that a shorter mean disease duration was a significant predictor of clinical remission in real-world observational studies evaluating CD, but not UC. These findings underscore the importance of early intervention with advanced therapy among patients with CD.
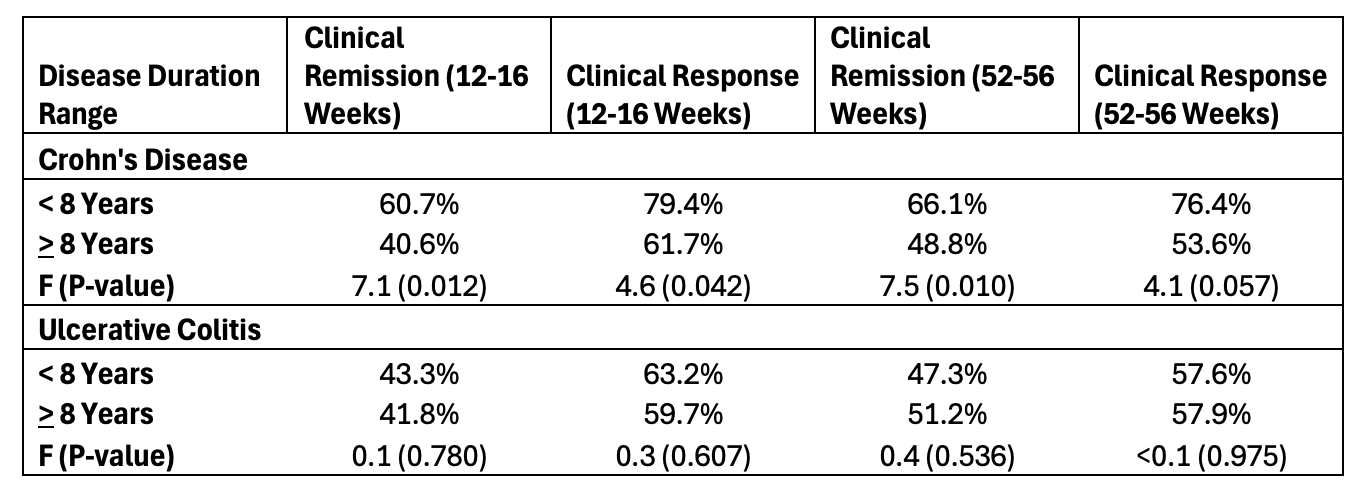
Figure: Table 1. Induction and maintenance phase clinical response and remission rates based on average study disease duration, stratified by Crohn’s Disease and Ulcerative Colitis.
Disclosures:
Michael Dunn indicated no relevant financial relationships.
John Haydek indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Eli Lilly – Grant/Research Support. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Michael Scott. Dunn, MD1, John P.. Haydek, MD, MS2, Edward L. Barnes, MD, MPH3. P5462 - Shorter Disease Duration Increases Clinical Remission Rates in Real-World Observational Studies for Crohn’s Disease, but Not Ulcerative Colitis: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1UNC School of Medicine, Chapel Hill, NC; 2Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, of North Carolina at Chapel Hill, Chapel Hill, NC; 3Multidisciplinary Inflammatory Bowel Diseases Center, Division of Gastroenterology and Hepatology, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA, Chapel Hill, NC
Introduction: Although previous clinical trials and observational studies have shown a correlation between early intervention with advanced therapies and improved clinical outcomes in inflammatory bowel disease, no comprehensive review has been performed. The goal of this review was to evaluate the effect of disease duration on clinical remission rates in real-world studies.
Methods: We searched Cochrane, Embase, PubMed, and Scopus for retrospective and prospective cohort studies on adult patients (>18 years) with Crohn’s Disease (CD) or Ulcerative Colitis (UC) treated with tumor necrosis factor (TNF) antagonists, anti-integrin, anti-interleukin (IL)-12 and IL-23 agents, Janus Kinase (JAK) inhibitors, or Sphingosine-1-Phosphate (S1P) receptor modulators. Data was extracted for mean disease duration at time of therapy initiation, clinical remission, and clinical response rates at the end of induction (week 12-16) and maintenance (week 52-56) phases of treatment. A meta-analysis with single-factor analysis of variation (ANOVA) was performed to analyze the correlation between disease duration, year of study publication, and clinical remission and response rates.
Results: The search strategy yielded 5,947 citations, of which 141 studies (83 UC, 64 CD) were eligible for inclusion. Among studies evaluating CD, participants with a shorter mean disease duration (< 8 years) compared to longer disease duration (> 8 years), achieved higher rates of clinical remission at the end of induction phase (60.7% vs 40.6%) and maintenance phase (66.1% vs 48.8%) (P < 0.05 for all comparisons, Table 1). In contrast, mean disease duration was not significantly associated with clinical remission in studies evaluating UC. In an exploratory analysis among patients with CD, there was no difference in clinical remission for induction phase or maintenance phase when comparing participants with a disease duration <5 years to those with a duration of 5-8 years (53.0% vs 62.6%) and (64.1% vs 68.1%) respectively.
Discussion: In this systematic review and meta-analysis, we demonstrated that a shorter mean disease duration was a significant predictor of clinical remission in real-world observational studies evaluating CD, but not UC. These findings underscore the importance of early intervention with advanced therapy among patients with CD.

Figure: Table 1. Induction and maintenance phase clinical response and remission rates based on average study disease duration, stratified by Crohn’s Disease and Ulcerative Colitis.
Disclosures:
Michael Dunn indicated no relevant financial relationships.
John Haydek indicated no relevant financial relationships.
Edward Barnes: AbbVie, Inc. – Consultant. Boomerang – Consultant. Eli Lilly – Consultant. Eli Lilly – Grant/Research Support. Pfizer – Consultant. Sanofi – Consultant. Takeda – Consultant. Target RWE – Consultant.
Michael Scott. Dunn, MD1, John P.. Haydek, MD, MS2, Edward L. Barnes, MD, MPH3. P5462 - Shorter Disease Duration Increases Clinical Remission Rates in Real-World Observational Studies for Crohn’s Disease, but Not Ulcerative Colitis: A Systematic Review and Meta-Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
