Tuesday Poster Session
Category: IBD
P5455 - Real-World Assessment of Risankizumab in Preventing Post-Operative Recurrence of Crohn’s Disease: A Comparison to Ustekinumab and Anti-TNF
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Jeremy A. Klein, MD (he/him/his)
University of Chicago Medicine, Inflammatory Bowel Disease Center
Chicago, IL
Presenting Author(s)
Award: ACG Presidential Poster Award
Jeremy A.. Klein, MD1, Russell Yanofsky, MD1, Meredith R. Kline, MD1, Aaron Goffinet, MD1, Asher Shafrir, MD1, Jessica Tanouye, 1, Alexandra R.. Hannett, 1, Tessa George, BSc1, Alex J. Mathew, MBE1, Alexandra McDermott, BSc1, David Choi, PharmD2, Amelia Kellar, MD, MSc1, David T. Rubin, MD3
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Post-operative recurrence (POR) of Crohn’s disease (CD) is common and current guidelines recommend postoperative therapy in high-risk patients. We evaluated the effectiveness of risankizumab (RZB) in POR of CD following ileal, colonic, or ileocolonic resection and compared it to ustekinumab (UST) and anti-tumor necrosis factor (TNF).
Methods: This is a retrospective review of prospectively collected real-world data from 2019-2025 of patients (pts) with CD who underwent surgical resection and initiated postoperative therapy with RZB, UST, or anti-TNF within 6 months (m) of surgery. The primary endpoint was endoscopic remission (defined as Rutgeerts < i2) within 6-12 m following surgery. Secondary outcomes included remission as measured by Harvey-Bradshaw Index ≤4, C-reactive protein < 3 mg/L or fecal calprotectin ≤150 μg/g), or cross-sectional imaging.
Results: Of 115 pts with CD (59% female) who underwent resection, the median age at diagnosis was 22.26 y [IQR 17.2-29.4], median age at surgery was 34.8 y [IQR 27.2-48.7], and median duration from diagnosis to surgery was 11.2 y [IQR 4.6-18.0]. Post-op treatments included RZB (n=35), UST (n=36), and anti-TNF (n=44) (Table 1). Most patients were advanced treatment experienced. There was no significant difference in endoscopic postoperative remission among RZB, UST, and anti-TNF therapies (RZB: 20 [74.1%], UST: 25 [83.2%], anti-TNF: 32 [86.5%]; p = 0.34 for RZB vs. anti-TNF, p = 0.36 for RZB vs. UST). There was no significant difference in POR-free survival among the three groups (p=0.41, Kaplan-Meyer, Figure 1). Cross-sectional imaging remission was more frequent with RZB compared to UST (12 [63.2%] vs. 2 [22.2%], p = 0.05), but not compared to anti-TNF (7 [63.6%], p = 1.00). In univariate and multivariate logistic regression of all patients, younger age at surgery (p = 0.03, OR 0.96) and prior use of 3 or more advanced therapies (p = 0.04, OR 0.32) were predictors of post-operative recurrence. Only one pt on RZB reported arthralgia as treatment-related AE.
Discussion: This is the first report of RZB for prevention of POR in CD. We found no significant differences in endoscopic POR in pts treated with RZB compared to UST or anti-TNF. Across all pts in this analysis, younger age at surgery and prior use of 3 or more advanced therapies were predictors of 6-12 m POR, underscoring the importance of post operative prophylaxis in high-risk patients. Our results suggest that RZB is an effective and safe option for post-operative prevention of CD.
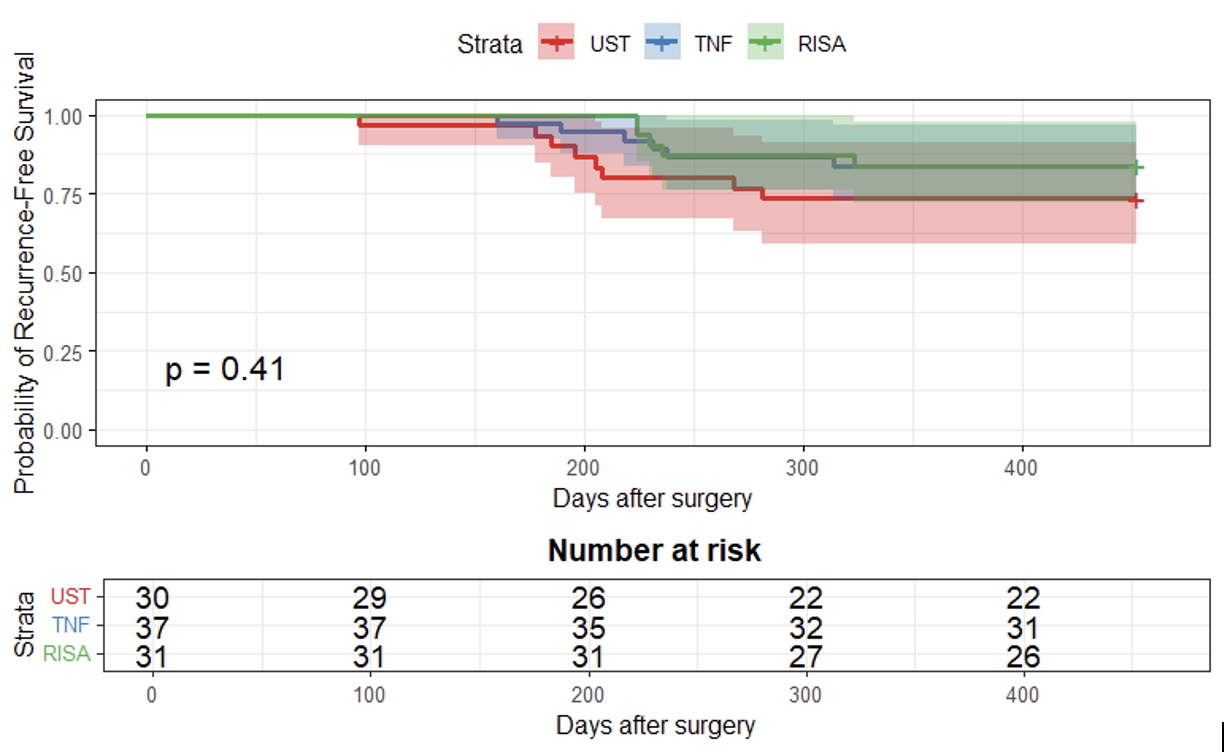
Figure: Figure 1. Kaplan-Meier curve with the probability of endoscopic recurrence-free survival in RZB versus UST versus Anti-TNF.
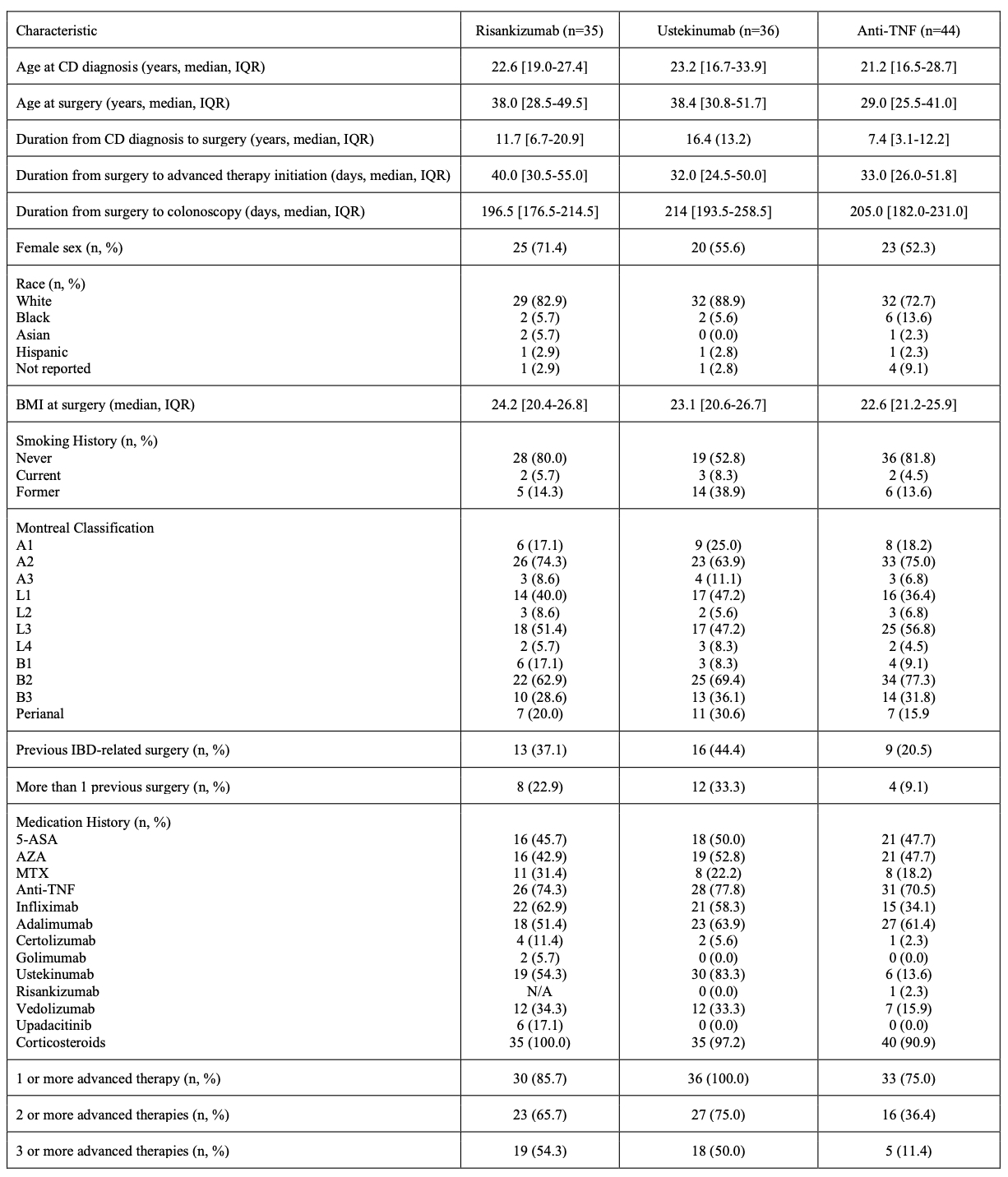
Figure: Table 1. Patient demographics and disease characteristics by treatment (RZB, UST, or Anti-TNF).
Disclosures:
Jeremy Klein indicated no relevant financial relationships.
Russell Yanofsky indicated no relevant financial relationships.
Meredith Kline indicated no relevant financial relationships.
Aaron Goffinet indicated no relevant financial relationships.
Asher Shafrir indicated no relevant financial relationships.
Jessica Tanouye indicated no relevant financial relationships.
Alexandra Hannett indicated no relevant financial relationships.
Tessa George indicated no relevant financial relationships.
Alex Mathew indicated no relevant financial relationships.
Alexandra McDermott indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Amelia Kellar indicated no relevant financial relationships.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Jeremy A.. Klein, MD1, Russell Yanofsky, MD1, Meredith R. Kline, MD1, Aaron Goffinet, MD1, Asher Shafrir, MD1, Jessica Tanouye, 1, Alexandra R.. Hannett, 1, Tessa George, BSc1, Alex J. Mathew, MBE1, Alexandra McDermott, BSc1, David Choi, PharmD2, Amelia Kellar, MD, MSc1, David T. Rubin, MD3. P5455 - Real-World Assessment of Risankizumab in Preventing Post-Operative Recurrence of Crohn’s Disease: A Comparison to Ustekinumab and Anti-TNF, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
Jeremy A.. Klein, MD1, Russell Yanofsky, MD1, Meredith R. Kline, MD1, Aaron Goffinet, MD1, Asher Shafrir, MD1, Jessica Tanouye, 1, Alexandra R.. Hannett, 1, Tessa George, BSc1, Alex J. Mathew, MBE1, Alexandra McDermott, BSc1, David Choi, PharmD2, Amelia Kellar, MD, MSc1, David T. Rubin, MD3
1University of Chicago Medicine, Inflammatory Bowel Disease Center, Chicago, IL; 2University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL; 3University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago, IL, USA, Chicago, IL
Introduction: Post-operative recurrence (POR) of Crohn’s disease (CD) is common and current guidelines recommend postoperative therapy in high-risk patients. We evaluated the effectiveness of risankizumab (RZB) in POR of CD following ileal, colonic, or ileocolonic resection and compared it to ustekinumab (UST) and anti-tumor necrosis factor (TNF).
Methods: This is a retrospective review of prospectively collected real-world data from 2019-2025 of patients (pts) with CD who underwent surgical resection and initiated postoperative therapy with RZB, UST, or anti-TNF within 6 months (m) of surgery. The primary endpoint was endoscopic remission (defined as Rutgeerts < i2) within 6-12 m following surgery. Secondary outcomes included remission as measured by Harvey-Bradshaw Index ≤4, C-reactive protein < 3 mg/L or fecal calprotectin ≤150 μg/g), or cross-sectional imaging.
Results: Of 115 pts with CD (59% female) who underwent resection, the median age at diagnosis was 22.26 y [IQR 17.2-29.4], median age at surgery was 34.8 y [IQR 27.2-48.7], and median duration from diagnosis to surgery was 11.2 y [IQR 4.6-18.0]. Post-op treatments included RZB (n=35), UST (n=36), and anti-TNF (n=44) (Table 1). Most patients were advanced treatment experienced. There was no significant difference in endoscopic postoperative remission among RZB, UST, and anti-TNF therapies (RZB: 20 [74.1%], UST: 25 [83.2%], anti-TNF: 32 [86.5%]; p = 0.34 for RZB vs. anti-TNF, p = 0.36 for RZB vs. UST). There was no significant difference in POR-free survival among the three groups (p=0.41, Kaplan-Meyer, Figure 1). Cross-sectional imaging remission was more frequent with RZB compared to UST (12 [63.2%] vs. 2 [22.2%], p = 0.05), but not compared to anti-TNF (7 [63.6%], p = 1.00). In univariate and multivariate logistic regression of all patients, younger age at surgery (p = 0.03, OR 0.96) and prior use of 3 or more advanced therapies (p = 0.04, OR 0.32) were predictors of post-operative recurrence. Only one pt on RZB reported arthralgia as treatment-related AE.
Discussion: This is the first report of RZB for prevention of POR in CD. We found no significant differences in endoscopic POR in pts treated with RZB compared to UST or anti-TNF. Across all pts in this analysis, younger age at surgery and prior use of 3 or more advanced therapies were predictors of 6-12 m POR, underscoring the importance of post operative prophylaxis in high-risk patients. Our results suggest that RZB is an effective and safe option for post-operative prevention of CD.

Figure: Figure 1. Kaplan-Meier curve with the probability of endoscopic recurrence-free survival in RZB versus UST versus Anti-TNF.

Figure: Table 1. Patient demographics and disease characteristics by treatment (RZB, UST, or Anti-TNF).
Disclosures:
Jeremy Klein indicated no relevant financial relationships.
Russell Yanofsky indicated no relevant financial relationships.
Meredith Kline indicated no relevant financial relationships.
Aaron Goffinet indicated no relevant financial relationships.
Asher Shafrir indicated no relevant financial relationships.
Jessica Tanouye indicated no relevant financial relationships.
Alexandra Hannett indicated no relevant financial relationships.
Tessa George indicated no relevant financial relationships.
Alex Mathew indicated no relevant financial relationships.
Alexandra McDermott indicated no relevant financial relationships.
David Choi: Abbvie – Advisory Committee/Board Member, Speakers Bureau. Boehringer – Advisory Committee/Board Member. Bristol Myers Squibb – Advisory Committee/Board Member. Eli Lilly – Advisory Committee/Board Member, Speakers Bureau. Johnson and Johnso – Advisory Committee/Board Member, Speakers Bureau. Pfizer – Advisory Committee/Board Member.
Amelia Kellar indicated no relevant financial relationships.
David Rubin: AbbVie – Advisory Committee/Board Member, Consultant, Speaker fees. Abivax SA – Consultant. Altrubio – Advisory Committee/Board Member, Consultant, Speaker feees, Stock Options. Avalo – Advisory Committee/Board Member, Consultant, Speaker fees. Bausch Health – Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker fees. Buhlmann Diagnostics – Advisory Committee/Board Member, Consultant, Speaker fees. Celltrion – Consultant. ClostraBio – Consultant. Connect BioPharma – Consultant. Cornerstones Health, Inc – Board of Directors membership. Douglas Pharmaceuticals – Consultant. Eli Lilly & Co. – Consultant. Foresee, Genentech (Roche) Inc. – Consultant. Image Analysis Group – Consultant. InDex Pharmaceutical – Consultant. Intouch Group – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Advisory Committee/Board Member, Consultant, Speaker fees. Iterative Health – Stock Options. Janssen Pharmaceuticals – Consultant. Lilly – Advisory Committee/Board Member, Consultant, Speaker fees. Odyssey Therapeutics – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker fees. Sanofi – Consultant. Takeda – Advisory Committee/Board Member, Consultant, Grant/Research Support, Speaker fees. Throne – Consultant. Vedanta – Consultant.
Jeremy A.. Klein, MD1, Russell Yanofsky, MD1, Meredith R. Kline, MD1, Aaron Goffinet, MD1, Asher Shafrir, MD1, Jessica Tanouye, 1, Alexandra R.. Hannett, 1, Tessa George, BSc1, Alex J. Mathew, MBE1, Alexandra McDermott, BSc1, David Choi, PharmD2, Amelia Kellar, MD, MSc1, David T. Rubin, MD3. P5455 - Real-World Assessment of Risankizumab in Preventing Post-Operative Recurrence of Crohn’s Disease: A Comparison to Ustekinumab and Anti-TNF, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

