Tuesday Poster Session
Category: IBD
P5446 - Is Tramadol a Safer Opioid in IBD? Insights From a Large Propensity-Matched Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Tarek Odah, MD, MPH
Mayo Clinic
Jacksonville, FL
Presenting Author(s)
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1
1Mayo Clinic, Jacksonville, FL; 2University of Missouri Kansas City School of Medicine, Kansas City, MO
Introduction: Opioid use in inflammatory bowel disease (IBD) is common but carries concerns regarding disease flares, complications, and masking of symptoms. While strong opioids have been linked to adverse outcomes, data on tramadol, a weak opioid agonist, remain limited.
Methods: We conducted a retrospective cohort study using the TriNetX platform to evaluate whether tramadol use is associated with disease activity in inflammatory bowel disease (IBD). Adults with ulcerative colitis (UC) or Crohn’s disease (CD) prescribed tramadol at least twice in the prior 6 months were compared to those prescribed hydrocodone, oxycodone, or codeine. Patients with a history of malignancy or who experienced the study outcomes prior to the index prescription were excluded. After 1:1 propensity score matching, 1,100 patients were included in each group. Covariates included demographics, IBD subtype, recent markers of disease severity (use of steroids, biologics, JAK inhibitors, or S1P receptor modulators), ER/inpatient encounters, psychiatric comorbidities, nicotine dependence, chronic pain, opioid-related disorders, and postoperative follow up visits. The primary outcome was a composite of new systemic steroid use (IV or oral), advanced therapy initiation, or fecal calprotectin ≥200 µg/g. Secondary outcomes included individual components, IBD-related surgery, ICD-based documentation of complications, ER/inpatient encounters, and mortality.
Results: After matching, baseline characteristics were well balanced as shown in Table 1. The composite outcome occurred in 16 (6.2%) vs. 24 (7.5%) patients (OR 0.82, 95% CI 0.43–1.58, p=0.56). IV steroid use occurred in 17 (2.8%) vs. 15 (2.2%) (OR 1.27, 95% CI 0.63–2.56, p=0.51), and oral steroid use in 22 (5.4%) vs. 31 (6.6%) (OR 0.82, 95% CI 0.46–1.43, p=0.48). ICD-coded complications were seen in ≤10 vs. 12, and ER/inpatient encounters in ≤10 vs. 13. Advanced therapy initiation, elevated fecal calprotectin (≥200 µg/g), and IBD-related surgery each occurred in ≤10 patients in both groups.
Discussion: In this large matched cohort of patients with inflammatory bowel disease, tramadol was associated with a similar risk of disease activity compared to other commonly prescribed oral opioids. These findings suggest tramadol may be a comparably safe option when opioid therapy is necessary, though opioids should always be used cautiously in this population.
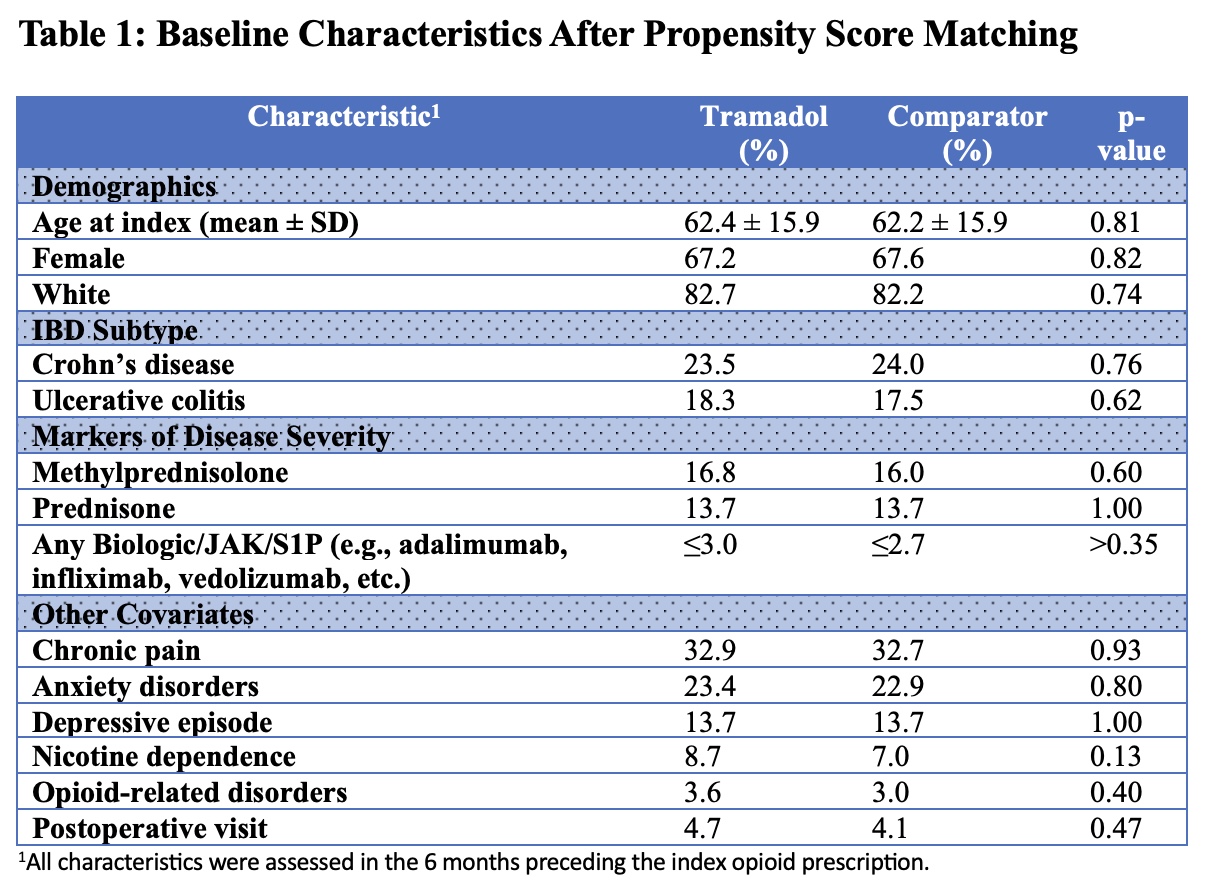
Figure: Table 1: Baseline Characteristics After Propensity Score Matching
Disclosures:
Tarek Odah indicated no relevant financial relationships.
Hassan Ghoz indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1. P5446 - Is Tramadol a Safer Opioid in IBD? Insights From a Large Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Mayo Clinic, Jacksonville, FL; 2University of Missouri Kansas City School of Medicine, Kansas City, MO
Introduction: Opioid use in inflammatory bowel disease (IBD) is common but carries concerns regarding disease flares, complications, and masking of symptoms. While strong opioids have been linked to adverse outcomes, data on tramadol, a weak opioid agonist, remain limited.
Methods: We conducted a retrospective cohort study using the TriNetX platform to evaluate whether tramadol use is associated with disease activity in inflammatory bowel disease (IBD). Adults with ulcerative colitis (UC) or Crohn’s disease (CD) prescribed tramadol at least twice in the prior 6 months were compared to those prescribed hydrocodone, oxycodone, or codeine. Patients with a history of malignancy or who experienced the study outcomes prior to the index prescription were excluded. After 1:1 propensity score matching, 1,100 patients were included in each group. Covariates included demographics, IBD subtype, recent markers of disease severity (use of steroids, biologics, JAK inhibitors, or S1P receptor modulators), ER/inpatient encounters, psychiatric comorbidities, nicotine dependence, chronic pain, opioid-related disorders, and postoperative follow up visits. The primary outcome was a composite of new systemic steroid use (IV or oral), advanced therapy initiation, or fecal calprotectin ≥200 µg/g. Secondary outcomes included individual components, IBD-related surgery, ICD-based documentation of complications, ER/inpatient encounters, and mortality.
Results: After matching, baseline characteristics were well balanced as shown in Table 1. The composite outcome occurred in 16 (6.2%) vs. 24 (7.5%) patients (OR 0.82, 95% CI 0.43–1.58, p=0.56). IV steroid use occurred in 17 (2.8%) vs. 15 (2.2%) (OR 1.27, 95% CI 0.63–2.56, p=0.51), and oral steroid use in 22 (5.4%) vs. 31 (6.6%) (OR 0.82, 95% CI 0.46–1.43, p=0.48). ICD-coded complications were seen in ≤10 vs. 12, and ER/inpatient encounters in ≤10 vs. 13. Advanced therapy initiation, elevated fecal calprotectin (≥200 µg/g), and IBD-related surgery each occurred in ≤10 patients in both groups.
Discussion: In this large matched cohort of patients with inflammatory bowel disease, tramadol was associated with a similar risk of disease activity compared to other commonly prescribed oral opioids. These findings suggest tramadol may be a comparably safe option when opioid therapy is necessary, though opioids should always be used cautiously in this population.

Figure: Table 1: Baseline Characteristics After Propensity Score Matching
Disclosures:
Tarek Odah indicated no relevant financial relationships.
Hassan Ghoz indicated no relevant financial relationships.
Jana Hashash: BMS – Ad Board.
Francis Farraye: Astellas – Advisory Committee/Board Member. Avalo – Advisory Committee/Board Member. Bausch – Advisory Committee/Board Member. BMS – Advisory Committee/Board Member. Braintree Labs – Advisory Committee/Board Member. Fresenius Kabi – Advisory Committee/Board Member. GI Reviewers – Independent Contractor. IBD Educational Group – Independent Contractor. Iterative Health – Advisory Committee/Board Member, Stock Options. Janssen – Advisory Committee/Board Member. Lilly – DSMB. Pfizer – Advisory Committee/Board Member. Pharmacosmos – Advisory Committee/Board Member. Sandoz – Advisory Committee/Board Member. Viatris – Advisory Committee/Board Member.
Tarek Odah, MD, MPH1, Hassan Ghoz, MD2, Jana G. Hashash, MD, MSc, FACG1, Francis A.. Farraye, MD, MSc, MACG1. P5446 - Is Tramadol a Safer Opioid in IBD? Insights From a Large Propensity-Matched Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
