Tuesday Poster Session
Category: IBD
P5440 - Effectiveness and Safety of Guselkumab versus Risankizumab in Ulcerative Colitis: A Real-World Comparative Outcomes Analysis
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Ali Osman, MD, MSCI Candidate
Washington University School of Medicine in St. Louis
Ballwin, MO
Presenting Author(s)
Ali Osman, MD, MSCI Candidate1, Barffour Maxwell, MD, PhD2, Yezaz Ghouri, MD3
1Washington University School of Medicine in St. Louis, Ballwin, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3SSM Health, Bridgeton, MO
Introduction: Guselkumab and risankizumab are IL-23 inhibitors increasingly used in the management of moderate to severe ulcerative colitis (UC). This study was an attempt to characterized the comparative benefits and risks of Guselkumab vs. risankizumab in UC patients.
Methods: We performed a retrospective cohort study using the TriNetX Global Collaborative Network database. Adults (≥16 years) with UC treated with Guselkumab (n=118) or risankizumab (n=504) were identified. Patients with Crohn’s disease, psoriasis, or other autoimmune indications for IL-23 inhibitors were excluded. Outcomes were assessed over a 30 to 365-day post-treatment window. Analyses included risk comparisons and Kaplan–Meier survival analysis.
Results: Guselkumab was associated with significantly lower 1-year corticosteroid use when compared to risankizumab (22.0% vs. 34.5%; risk difference: -12.5%, 95% CI: -21.0 to -3.9; p=0.009). Kaplan–Meier estimates demonstrated a trend toward improved steroid-free survival with Guselkumab (55.9% vs. 39.8%; p=0.405). Rates of hospitalization or emergency department visits were similar between the two groups (8.5% vs. 10.7%; p=0.471). Advancement to other therapies, including anti-TNF, JAK inhibitors, or vedolizumab, occurred in 8.5% of the GUS group versus 7.3% of the risankizumab group (p=0.675). One of the limitations was that in this database, outcome counts fewer than 10 are rounded up to 10 to protect patient privacy. As a result, outcome rates below or equal to 8.5% may be overestimated, particularly in smaller cohorts.
Discussion: In this real-world analysis of patients with UC, guselkumab was associated with lower corticosteroid use when compared to risankizumab. Rates of hospitalization and therapy escalation were comparable between the two treatment groups. These findings suggest that Guselkumab may be a viable treatment option pending further validation in matched or prospective analyses.
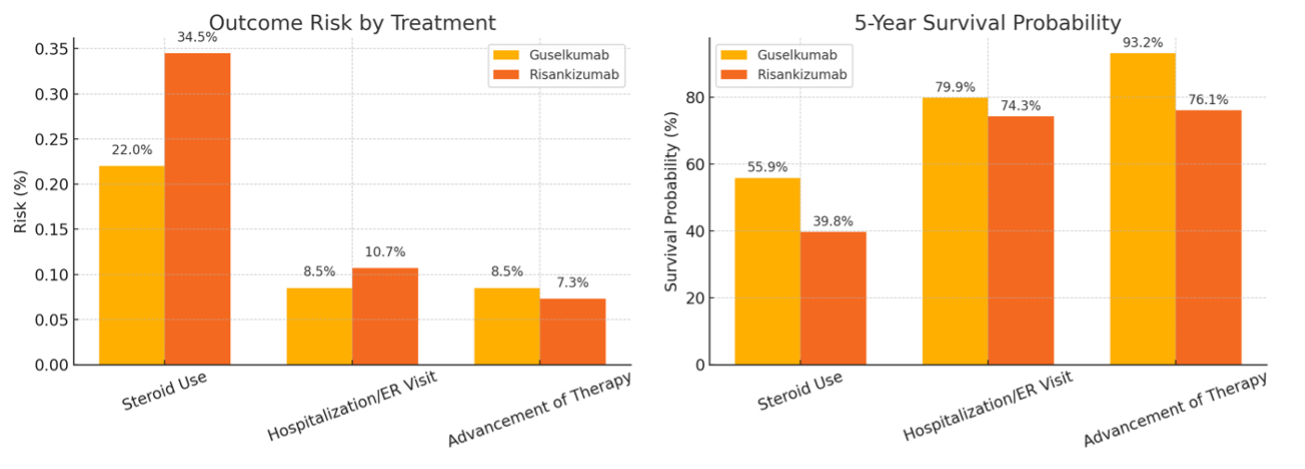
Figure: Figure. One-year risk of steroid use, hospitalization/ER visit, and advancement of therapy, as well as survival probability at 1 year, in patients with ulcerative colitis treated with Guselkumab vs. Risankizumab.
Disclosures:
Ali Osman indicated no relevant financial relationships.
Barffour Maxwell indicated no relevant financial relationships.
Yezaz Ghouri: Capsovision – Consultant. Celltrion – Speakers Bureau.
Ali Osman, MD, MSCI Candidate1, Barffour Maxwell, MD, PhD2, Yezaz Ghouri, MD3. P5440 - Effectiveness and Safety of Guselkumab versus Risankizumab in Ulcerative Colitis: A Real-World Comparative Outcomes Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Washington University School of Medicine in St. Louis, Ballwin, MO; 2Washington University School of Medicine in St. Louis, St. Louis, MO; 3SSM Health, Bridgeton, MO
Introduction: Guselkumab and risankizumab are IL-23 inhibitors increasingly used in the management of moderate to severe ulcerative colitis (UC). This study was an attempt to characterized the comparative benefits and risks of Guselkumab vs. risankizumab in UC patients.
Methods: We performed a retrospective cohort study using the TriNetX Global Collaborative Network database. Adults (≥16 years) with UC treated with Guselkumab (n=118) or risankizumab (n=504) were identified. Patients with Crohn’s disease, psoriasis, or other autoimmune indications for IL-23 inhibitors were excluded. Outcomes were assessed over a 30 to 365-day post-treatment window. Analyses included risk comparisons and Kaplan–Meier survival analysis.
Results: Guselkumab was associated with significantly lower 1-year corticosteroid use when compared to risankizumab (22.0% vs. 34.5%; risk difference: -12.5%, 95% CI: -21.0 to -3.9; p=0.009). Kaplan–Meier estimates demonstrated a trend toward improved steroid-free survival with Guselkumab (55.9% vs. 39.8%; p=0.405). Rates of hospitalization or emergency department visits were similar between the two groups (8.5% vs. 10.7%; p=0.471). Advancement to other therapies, including anti-TNF, JAK inhibitors, or vedolizumab, occurred in 8.5% of the GUS group versus 7.3% of the risankizumab group (p=0.675). One of the limitations was that in this database, outcome counts fewer than 10 are rounded up to 10 to protect patient privacy. As a result, outcome rates below or equal to 8.5% may be overestimated, particularly in smaller cohorts.
Discussion: In this real-world analysis of patients with UC, guselkumab was associated with lower corticosteroid use when compared to risankizumab. Rates of hospitalization and therapy escalation were comparable between the two treatment groups. These findings suggest that Guselkumab may be a viable treatment option pending further validation in matched or prospective analyses.

Figure: Figure. One-year risk of steroid use, hospitalization/ER visit, and advancement of therapy, as well as survival probability at 1 year, in patients with ulcerative colitis treated with Guselkumab vs. Risankizumab.
Disclosures:
Ali Osman indicated no relevant financial relationships.
Barffour Maxwell indicated no relevant financial relationships.
Yezaz Ghouri: Capsovision – Consultant. Celltrion – Speakers Bureau.
Ali Osman, MD, MSCI Candidate1, Barffour Maxwell, MD, PhD2, Yezaz Ghouri, MD3. P5440 - Effectiveness and Safety of Guselkumab versus Risankizumab in Ulcerative Colitis: A Real-World Comparative Outcomes Analysis, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
