Tuesday Poster Session
Category: IBD
P5432 - Early Symptomatic Improvement Associations With Week 12 Outcomes: E-Diary Results From the Etrasimod ELEVATE UC Clinical Program
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Remo Panaccione, MD (he/him/his)
Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada
Calgary, AB, Canada
Presenting Author(s)
Marla C. Dubinsky, MD1, María Chaparro, MD2, Peter M. Irving, MD3, Peter Hur, PharmD4, Sarah Sidhu, PhD5, John C. Woolcott, PhD6, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS Princesa), Universidad Autónoma de Madrid (UAM), Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain, Madrid, Madrid, Spain; 3IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 4Pfizer Inc, New York, NY; 5Pfizer Inc, New York, NY, USA, New York, NY; 6Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 7Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 8Division of Gastroenterology, Hospital Beatriz Ângelo, Loures, Portugal; Division of Gastroenterology, Hospital da Luz, Lisbon, Portugal; Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). Early patient (pt)-reported response is often seen as an indicator of continued improvement through Week (Wk) 12.1
Methods: This post hoc analysis investigated relationships between pt-reported symptoms by Day 7 and clinical endpoints at Wk 12 in pts receiving etrasimod 2 mg QD or placebo in the ELEVATE UC clinical program (NCT03945188, NCT03996369) using rectal bleeding (RB) and stool frequency (SF) subscores reported via daily e-diary records. For pts reporting symptomatic, RB, and SF response by Day 7 (yes/no), the proportion achieving clinical endpoints (clinical remission [CR], endoscopic improvement [EI], and EI-histologic remission [EIHR]) at Wk 12 was measured. Adjusted risk differences in proportions of responders between treatment groups were estimated and tested using the Mantel–Haenszel weighted method.
Results: Among pts reporting symptomatic response by Day 7 (etrasimod 2 mg, N1 = 151; placebo, N1 = 60), pts treated with etrasimod vs placebo demonstrated significantly greater CR (47.7% vs 15.0%, adjusted difference [Δ] 33.9, p < 0.001), EI (54.3% vs 20.0%, Δ 35.7, p < 0.001), and EIHR (31.1% vs 6.7%, Δ 24.8, p < 0.001) rates at Wk 12 (Table). In pts who did not demonstrate symptomatic response by Day 7 (etrasimod 2 mg, N1 = 376; placebo, N1 = 200), those treated with etrasimod vs placebo achieved significantly greater rates of CR (18.9% vs 10.0%, Δ 8.7, p = 0.002), EI (27.7% vs 17.0%, Δ 10.5, p = 0.002), and EIHR (16.0% vs 7.5%, Δ 8.3, p = 0.002) at Wk 12 (Table). Similarly, Day 7 RB and SF responders and nonresponders treated with etrasimod vs placebo also demonstrated significantly greater rates of CR, EI and EIHR at Wk 12 (all p < 0.001; Table).
Discussion: Pts treated with etrasimod may experience rapid symptomatic improvements. In ELEVATE UC, pts treated with etrasimod who both did and did not demonstrate rapid symptomatic improvements had higher rates of clinical endpoint achievement at Wk 12 vs placebo. Pts treated with etrasimod who demonstrated an early, Day 7 response had the greatest likelihood of meeting clinical endpoints at Wk 12.
Reference:
1. Chaparro M et al. J Crohns Colitis 2023; 17: i107–i110.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.
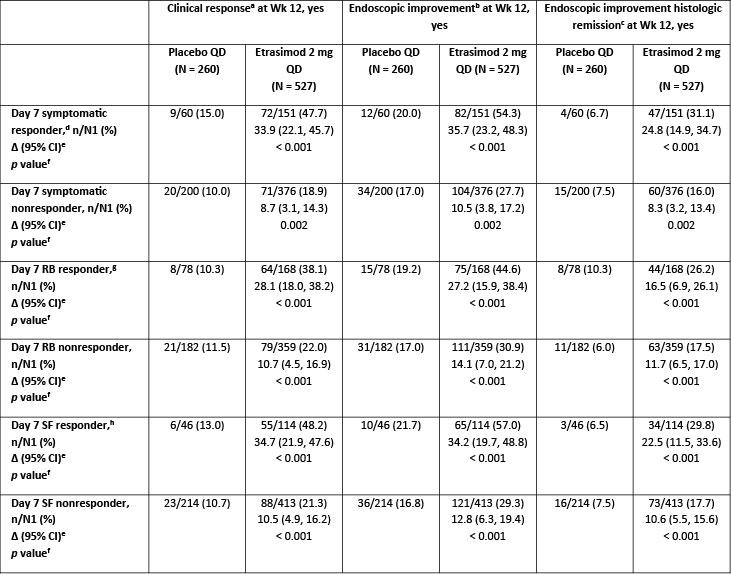
Figure: Table. Wk 12 clinical endpoint in Day 7 symptomatic, RB and SF responders and nonresponders (FAS with baseline MMS 4–9)
Data are pooled from ELEVATE UC 52 and ELEVATE UC 12.
[a]Defined as SF subscore = 0 (or = 1 with a ≥ 1-point decrease from baseline), RB subscore = 0, and endoscopic subscore ≤ 1 (excluding friability). SF and RB subscores are derived from pt daily e-diary records using the scores from the three most recent consecutive days within the seven days prior to the day of bowel preparation, averaged and rounded to the nearest integer.
[b]Defined as endoscopic subscore ≤ 1.
[c]Defined as endoscopic subscore ≤ 1 with histologic remission measured by a Geboes Index score < 2.0.
[d]Defined as pts achieving ≥ 30% decrease from baseline in partial MMS by Day 7.
[e]Difference (%) is based on estimated common risk difference using the Mantel–Haenszel weights and is stratified by actual naïve to biologic/Janus kinase inhibitor therapy at study entry (yes/no), actual baseline corticosteroid use (yes/no), and actual baseline disease activity (MMS: 4–6 or 7–9).
[f]The 2-sided p value is to test the hypothesis of the common risk difference being 0 using Mantel–Haenszel weighted test.
[g]Defined as pts achieving RB subscore ≥ 1-point decrease from baseline by Day 7, based on collected daily scores.
[h]Defined as pts achieving SF subscore = 0 or ≥ 1-point decrease from baseline by Day 7, based on collected daily scores.
Δ, adjusted difference; CI, confidence interval; CR, clinical remission; EI, endoscopic improvement; EIHR, EI-histologic remission; FAS, full analysis set; MMS, modified Mayo score; N, number of pts in treatment group; n, number of pts achieving Day 7 and Wk 12 endpoint; N1, number of pts achieving Day 7 endpoint; pt, patient; QD, once daily; RB, rectal bleeding; SF, stool frequency; UC, ulcerative colitis; Wk, Week.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
María Chaparro: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Dr Falk Pharma – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. Hospira – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Lilly – Consultant, Grant/Research Support, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Shire Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tillotts – Consultant, Grant/Research Support, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Sarah Sidhu: Pfizer Inc – Employee, Stock Options.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Wenjin Wang: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Lilly – Advisory Committee/Board Member, Speakers Bureau. Pfizer Inc – Advisory Committee/Board Member, Speakers Bureau. Takeda – Speakers Bureau.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Marla C. Dubinsky, MD1, María Chaparro, MD2, Peter M. Irving, MD3, Peter Hur, PharmD4, Sarah Sidhu, PhD5, John C. Woolcott, PhD6, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9. P5432 - Early Symptomatic Improvement Associations With Week 12 Outcomes: E-Diary Results From the Etrasimod ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Susan and Leonard Feinstein IBD Center, Icahn School of Medicine at Mount Sinai, New York, NY, USA, New York, NY; 2Department of Gastroenterology, Hospital Universitario de La Princesa, Instituto de Investigación Sanitaria Princesa (IIS Princesa), Universidad Autónoma de Madrid (UAM), Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Madrid, Spain, Madrid, Madrid, Spain; 3IBD Unit, Guy’s and St Thomas’ Hospital, London, UK, London, England, United Kingdom; 4Pfizer Inc, New York, NY; 5Pfizer Inc, New York, NY, USA, New York, NY; 6Pfizer Inc, Collegeville, PA, USA, Collegeville, PA; 7Pfizer AG, Zürich, Switzerland, Zürich, Zurich, Switzerland; 8Division of Gastroenterology, Hospital Beatriz Ângelo, Loures, Portugal; Division of Gastroenterology, Hospital da Luz, Lisbon, Portugal; Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal, Lisbon, Lisboa, Portugal; 9Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, Department of Medicine, University of Calgary, Calgary, AB, Canada, Calgary, AB, Canada
Introduction: Etrasimod is an oral, once-daily (QD), selective sphingosine 1‑phosphate (S1P)1,4,5 receptor modulator for the treatment of moderately to severely active ulcerative colitis (UC). Early patient (pt)-reported response is often seen as an indicator of continued improvement through Week (Wk) 12.1
Methods: This post hoc analysis investigated relationships between pt-reported symptoms by Day 7 and clinical endpoints at Wk 12 in pts receiving etrasimod 2 mg QD or placebo in the ELEVATE UC clinical program (NCT03945188, NCT03996369) using rectal bleeding (RB) and stool frequency (SF) subscores reported via daily e-diary records. For pts reporting symptomatic, RB, and SF response by Day 7 (yes/no), the proportion achieving clinical endpoints (clinical remission [CR], endoscopic improvement [EI], and EI-histologic remission [EIHR]) at Wk 12 was measured. Adjusted risk differences in proportions of responders between treatment groups were estimated and tested using the Mantel–Haenszel weighted method.
Results: Among pts reporting symptomatic response by Day 7 (etrasimod 2 mg, N1 = 151; placebo, N1 = 60), pts treated with etrasimod vs placebo demonstrated significantly greater CR (47.7% vs 15.0%, adjusted difference [Δ] 33.9, p < 0.001), EI (54.3% vs 20.0%, Δ 35.7, p < 0.001), and EIHR (31.1% vs 6.7%, Δ 24.8, p < 0.001) rates at Wk 12 (Table). In pts who did not demonstrate symptomatic response by Day 7 (etrasimod 2 mg, N1 = 376; placebo, N1 = 200), those treated with etrasimod vs placebo achieved significantly greater rates of CR (18.9% vs 10.0%, Δ 8.7, p = 0.002), EI (27.7% vs 17.0%, Δ 10.5, p = 0.002), and EIHR (16.0% vs 7.5%, Δ 8.3, p = 0.002) at Wk 12 (Table). Similarly, Day 7 RB and SF responders and nonresponders treated with etrasimod vs placebo also demonstrated significantly greater rates of CR, EI and EIHR at Wk 12 (all p < 0.001; Table).
Discussion: Pts treated with etrasimod may experience rapid symptomatic improvements. In ELEVATE UC, pts treated with etrasimod who both did and did not demonstrate rapid symptomatic improvements had higher rates of clinical endpoint achievement at Wk 12 vs placebo. Pts treated with etrasimod who demonstrated an early, Day 7 response had the greatest likelihood of meeting clinical endpoints at Wk 12.
Reference:
1. Chaparro M et al. J Crohns Colitis 2023; 17: i107–i110.
Pfizer’s generative artificial intelligence tool MAIA was used to assist production of the abstract first draft. Authors reviewed/edited and take responsibility for the content.

Figure: Table. Wk 12 clinical endpoint in Day 7 symptomatic, RB and SF responders and nonresponders (FAS with baseline MMS 4–9)
Data are pooled from ELEVATE UC 52 and ELEVATE UC 12.
[a]Defined as SF subscore = 0 (or = 1 with a ≥ 1-point decrease from baseline), RB subscore = 0, and endoscopic subscore ≤ 1 (excluding friability). SF and RB subscores are derived from pt daily e-diary records using the scores from the three most recent consecutive days within the seven days prior to the day of bowel preparation, averaged and rounded to the nearest integer.
[b]Defined as endoscopic subscore ≤ 1.
[c]Defined as endoscopic subscore ≤ 1 with histologic remission measured by a Geboes Index score < 2.0.
[d]Defined as pts achieving ≥ 30% decrease from baseline in partial MMS by Day 7.
[e]Difference (%) is based on estimated common risk difference using the Mantel–Haenszel weights and is stratified by actual naïve to biologic/Janus kinase inhibitor therapy at study entry (yes/no), actual baseline corticosteroid use (yes/no), and actual baseline disease activity (MMS: 4–6 or 7–9).
[f]The 2-sided p value is to test the hypothesis of the common risk difference being 0 using Mantel–Haenszel weighted test.
[g]Defined as pts achieving RB subscore ≥ 1-point decrease from baseline by Day 7, based on collected daily scores.
[h]Defined as pts achieving SF subscore = 0 or ≥ 1-point decrease from baseline by Day 7, based on collected daily scores.
Δ, adjusted difference; CI, confidence interval; CR, clinical remission; EI, endoscopic improvement; EIHR, EI-histologic remission; FAS, full analysis set; MMS, modified Mayo score; N, number of pts in treatment group; n, number of pts achieving Day 7 and Wk 12 endpoint; N1, number of pts achieving Day 7 endpoint; pt, patient; QD, once daily; RB, rectal bleeding; SF, stool frequency; UC, ulcerative colitis; Wk, Week.
Disclosures:
Marla Dubinsky: AbbVie – Consultant, Grant/Research Support. Arena – Consultant. Astra Zeneca – Consultant. Boehringer Ingelheim – Consultant. Celgene – Consultant. Eli Lilly – Consultant. Gilead – Consultant. Janssen – Consultant, Grant/Research Support. Merck – Consultant. Pfizer – Consultant. Prometheus Laboratories – Consultant, Grant/Research Support. Sanofi – Consultant. Spyre – Consultant. Takeda – Consultant. Trellus Health – Stock Options, Stock-publicly held company(excluding mutual/index funds). UCB – Consultant.
María Chaparro: AbbVie – Consultant, Grant/Research Support, Speakers Bureau. Biogen – Consultant, Grant/Research Support, Speakers Bureau. Dr Falk Pharma – Consultant, Grant/Research Support, Speakers Bureau. Ferring – Consultant, Grant/Research Support, Speakers Bureau. Gilead – Consultant, Grant/Research Support, Speakers Bureau. Hospira – Consultant, Grant/Research Support, Speakers Bureau. Janssen – Consultant, Grant/Research Support, Speakers Bureau. Lilly – Consultant, Grant/Research Support, Speakers Bureau. MSD – Consultant, Grant/Research Support, Speakers Bureau. Pfizer Inc – Consultant, Grant/Research Support, Speakers Bureau. Shire Pharmaceuticals – Consultant, Grant/Research Support, Speakers Bureau. Takeda – Consultant, Grant/Research Support, Speakers Bureau. Tillotts – Consultant, Grant/Research Support, Speakers Bureau.
Peter Irving: Arena – Advisor or Review Panel Member. Boehringer Ingelheim – Advisor or Review Panel Member. Bristol Myers Squibb – Advisory Committee/Board Member, Speakers Bureau. Celgene – Advisor or Review Panel Member, Speakers Bureau. Celltrion – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Falk Pharma – Speakers Bureau. Ferring – Speakers Bureau. Galapagos – Speakers Bureau. Genentech – Advisor or Review Panel Member. Gilead – Advisor or Review Panel Member, Speakers Bureau. Hospira – Advisor or Review Panel Member. Janssen – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. MSD – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Pfizer Inc – Advisor or Review Panel Member, Speakers Bureau. Pharmacosmos – Advisor or Review Panel Member. Prometheus – Advisor or Review Panel Member. Roche – Advisor or Review Panel Member. Samsung Bioepis – Advisor or Review Panel Member. Sandoz – Advisor or Review Panel Member, Speakers Bureau. Sapphire Medical – Speakers Bureau. Shire – Speakers Bureau. Takeda – Advisor or Review Panel Member, Grant/Research Support, Speakers Bureau. Tillotts – Speakers Bureau. Topivert – Advisor or Review Panel Member. VH2 – Advisor or Review Panel Member. Vifor Pharma – Advisor or Review Panel Member. Warner Chilcott – Advisor or Review Panel Member, Speakers Bureau.
Peter Hur: AbbVie – Grant/Research Support. Bristol Myers Squibb – Grant/Research Support. Buhlmann – Grant/Research Support. Clene Nanomedicine – Stock Options. Haleon – Stock Options. Idorsia – Stock Options. Janssen – Grant/Research Support. Lilly – Grant/Research Support. Liquidia – Stock Options. Longboard Pharmaceuticals – Stock Options. Pfizer Inc – Employee, Grant/Research Support, Stock Options. Proctor & Gamble – Stock Options. Takeda – Grant/Research Support. US 2022/0257594 A1 – Intellectual Property/Patents.
Sarah Sidhu: Pfizer Inc – Employee, Stock Options.
John Woolcott: Pfizer Inc – Employee, Stock Options.
Wenjin Wang: Pfizer Inc – Employee, Stock Options.
Martina Goetsch: Pfizer AG – Employee. Pfizer Inc – Stock Options.
Joana Torres: AbbVie – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Janssen – Advisory Committee/Board Member, Grant/Research Support, Speakers Bureau. Lilly – Advisory Committee/Board Member, Speakers Bureau. Pfizer Inc – Advisory Committee/Board Member, Speakers Bureau. Takeda – Speakers Bureau.
Remo Panaccione: Abbott – Consultant. AbbVie – Advisory Committee/Board Member, Consultant, Speaker's fees. Abivax – Consultant. Alimentiv – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant, Speaker's fees. AnaptysBio – Consultant. Arena Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. AstraZeneca – Advisory Committee/Board Member, Consultant. Biogen – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim – Advisory Committee/Board Member, Consultant. Bristol Myers Squibb – Advisory Committee/Board Member, Consultant, Speaker's fees. Celgene – Advisory Committee/Board Member, Consultant, Speaker's fees. Celltrion – Consultant. Cosmos Pharmaceuticals – Consultant. Eisai – Consultant. Elan Pharmaceuticals – Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant, Speaker's fees. Ferring Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Fresenius Kabi – Advisory Committee/Board Member, Consultant, Speaker's fees. Galapagos – Consultant. Genentech (Roche) – Advisory Committee/Board Member, Consultant. Gilead Sciences – Advisory Committee/Board Member, Consultant, Speaker's fees. GlaxoSmithKline – Advisory Committee/Board Member, Consultant. JAMP Pharma – Advisory Committee/Board Member, Consultant. Johnson & Johnson – Advisory Committee/Board Member, Consultant, Speaker's fees. Merck & Co., Inc. – Advisory Committee/Board Member, Consultant, Speaker's fees. Mirador – Consultant. Mylan – Advisory Committee/Board Member, Consultant. Novartis – Advisory Committee/Board Member, Consultant. Odyssey – Consultant. Oppilan Pharma – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant, Speaker's fees. Pandion Therapeutics – Advisory Committee/Board Member, Consultant. Pendopharm G.I. Solutions – Consultant. Pfizer – Advisory Committee/Board Member, Consultant, Speaker's fees. Progenity – Advisory Committee/Board Member, Consultant. Prometheus Biosciences – Consultant. Protagonist Therapeutics Inc – Advisory Committee/Board Member, Consultant. Roche – Advisory Committee/Board Member, Consultant, Speaker's fees. Sandoz – Advisory Committee/Board Member, Consultant, Speaker's fees. Sanofi – Consultant. Satisfai Health – Consultant. Shire Pharma – Advisory Committee/Board Member, Consultant, Speaker's fees. Spyre Therapeutics – Consultant. Sublimity Therapeutics – Advisory Committee/Board Member, Consultant. Takeda Pharmaceuticals – Advisory Committee/Board Member, Consultant, Speaker's fees. Teva – Consultant. Theravance Biopharma – Consultant. Tillots – Consultant. Trellus Health – Consultant. UCB – Consultant. Union Biopharma – Consultant. Ventyx Biosciences – Advisory Committee/Board Member, Consultant. Viatris – Consultant.
Marla C. Dubinsky, MD1, María Chaparro, MD2, Peter M. Irving, MD3, Peter Hur, PharmD4, Sarah Sidhu, PhD5, John C. Woolcott, PhD6, Wenjin Wang, PhD6, Martina Goetsch, MD7, Joana Torres, MD, PhD8, Remo Panaccione, MD9. P5432 - Early Symptomatic Improvement Associations With Week 12 Outcomes: E-Diary Results From the Etrasimod ELEVATE UC Clinical Program, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
