Tuesday Poster Session
Category: IBD
P5395 - Efficacy of 5-ASA Continuation versus Discontinuation in Patients With Moderate to Severe Ulcerative Colitis Escalated to Advanced Therapy: A Systematic Review and Meta-Analysis of Adjusted Effect Estimates
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Hyun Hee Sul, MD (she/her/hers)
Jersey City Medical Center
Jersey City , NJ
Presenting Author(s)
Hyun Hee Sul, MD1, Douglas Mesadri. Gewehr, MD2, Hara Kang, MD3, Odery Ramos jr, PhD4
1Jersey City Medical Center, Fort Lee, NJ; 2Federal University of Paraná, Curitiba, Parana, Brazil; 3Samsung Medical Center, Paramus, NJ; 4Universidade Federal do Paraná - Brazil, Curitiba, Parana, Brazil
Introduction: The benefit of continuing 5-aminosalicylates (5-ASA) in patients with moderate-to-severe ulcerative colitis (UC) escalated to advanced therapies remains uncertain. To address this issue, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of 5-ASA continuation versus discontinuation in this population.
Methods: We systematically searched PubMed, Embase, and Cochrane databases for studies comparing continuation versus discontinuation of 5-ASA in adult patients with moderate-to-severe UC escalated to advanced therapies. We attempted to use multivariable-adjusted data to account for known confounding factors. Adjusted effect estimates were pooled using inverse-variance random-effects models for adjusted odds ratios (aORs) and hazard ratios (aHRs). Risk of bias was assessed using the ROBINS-I tool.
Results: Our meta-analysis comprised nine observational cohorts and three studies presenting post hoc analyses from twelve clinical trials. We included 16,461 patients, with 12,652 assigned to 5-ASA continuation and 3,809 to 5-ASA discontinuation. Compared to discontinuation, 5-ASA continuation was associated with decreased odds of achieving clinical remission (aOR 0.72; 95% CI 0.52-0.99; p=0.046; I2=0%). There were no significant differences between groups for corticosteroid-free clinical remission (aOR 0.71; 95% CI 0.41-1.23; p=0.22; I2=0%), clinical response (aOR 0.94; 95% CI 0.69-1.28; p=0.70; I2=0%), endoscopic healing (OR 0.96; 95% CI 0.64-1.44; p=0.85; I2=61%), and biochemical remission (aOR 1.13; 95% CI 0.76-1.68; p=0.54; I2=31%). In time-to-event analyses, no significant differences were found between groups for UC-related hospitalization (aHR 1.08; 95% CI 0.89-1.31; p=0.42; I2=51%), UC-related surgery (aHR 0.90; 95% CI 0.71-1.15; p=0.41; I2=36%), new corticosteroid prescription (aHR 0.92; 95% CI 0.79-1.06; p=0.24; I2=77%), composite adverse events (aHR 0.96; 95% CI 0.87-1.06; p=0.43; I2=0%), and loss of response (aHR 0.90; 95% CI 0.76-1.07; p=0.25; I2=77%). ROBINS-I results found one study with a serious risk of bias and eleven with a moderate risk of bias.
Discussion: In this meta-analysis of adjusted effect estimates, 5-ASA continuation was associated with a decreased odds of achieving clinical remission compared with 5-ASA discontinuation, with no significant differences observed for other efficacy and safety endpoints. These findings suggest that 5-ASA may be safely discontinued after escalation to advanced therapies in this population.
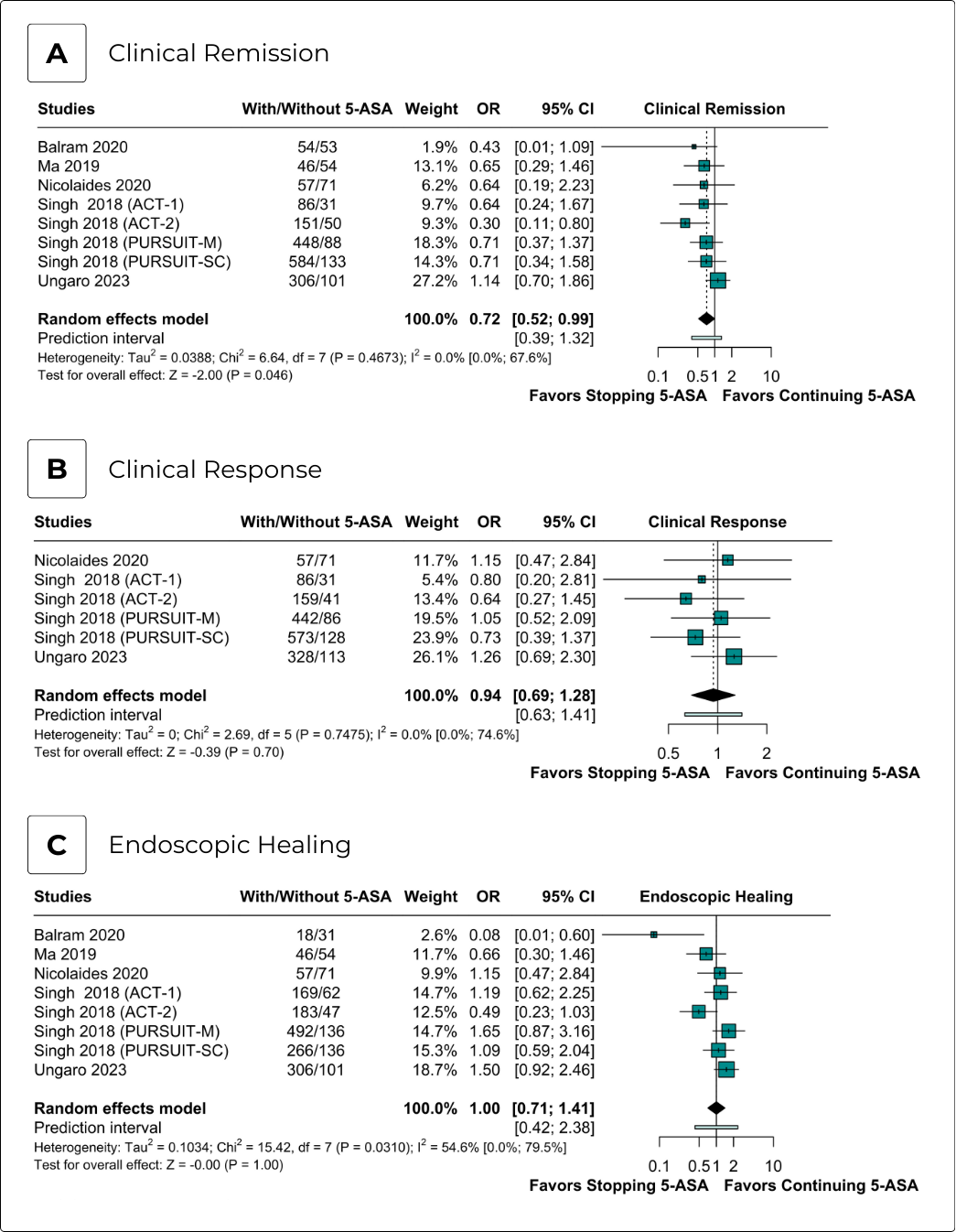
Figure: Efficacy Endpoints
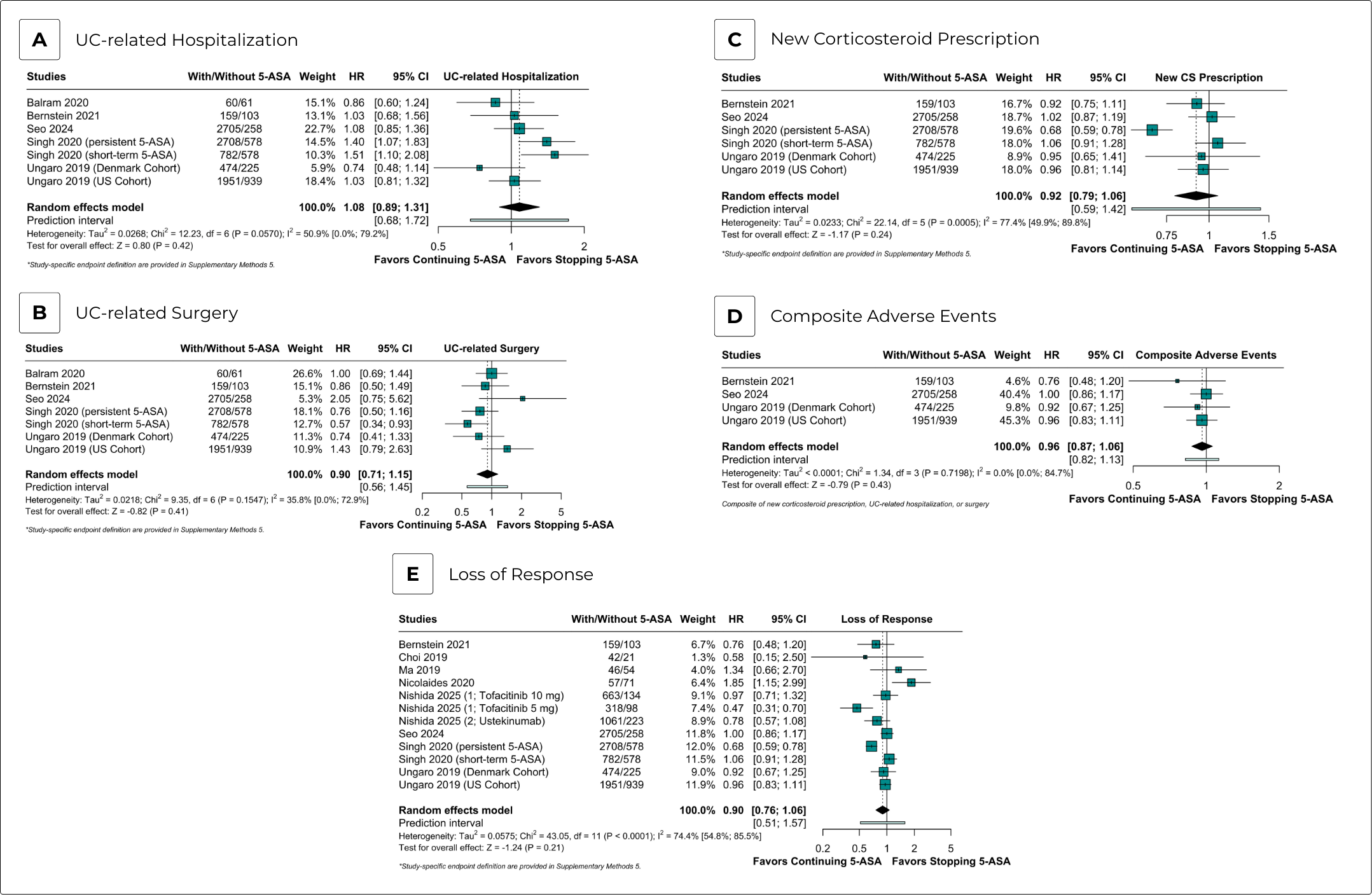
Figure: Safety Endpoints - Time-to-Event Analysis
Disclosures:
Hyun Hee Sul indicated no relevant financial relationships.
Douglas Gewehr indicated no relevant financial relationships.
Hara Kang indicated no relevant financial relationships.
Odery Ramos jr indicated no relevant financial relationships.
Hyun Hee Sul, MD1, Douglas Mesadri. Gewehr, MD2, Hara Kang, MD3, Odery Ramos jr, PhD4. P5395 - Efficacy of 5-ASA Continuation versus Discontinuation in Patients With Moderate to Severe Ulcerative Colitis Escalated to Advanced Therapy: A Systematic Review and Meta-Analysis of Adjusted Effect Estimates, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Jersey City Medical Center, Fort Lee, NJ; 2Federal University of Paraná, Curitiba, Parana, Brazil; 3Samsung Medical Center, Paramus, NJ; 4Universidade Federal do Paraná - Brazil, Curitiba, Parana, Brazil
Introduction: The benefit of continuing 5-aminosalicylates (5-ASA) in patients with moderate-to-severe ulcerative colitis (UC) escalated to advanced therapies remains uncertain. To address this issue, we conducted a systematic review and meta-analysis to evaluate the efficacy and safety of 5-ASA continuation versus discontinuation in this population.
Methods: We systematically searched PubMed, Embase, and Cochrane databases for studies comparing continuation versus discontinuation of 5-ASA in adult patients with moderate-to-severe UC escalated to advanced therapies. We attempted to use multivariable-adjusted data to account for known confounding factors. Adjusted effect estimates were pooled using inverse-variance random-effects models for adjusted odds ratios (aORs) and hazard ratios (aHRs). Risk of bias was assessed using the ROBINS-I tool.
Results: Our meta-analysis comprised nine observational cohorts and three studies presenting post hoc analyses from twelve clinical trials. We included 16,461 patients, with 12,652 assigned to 5-ASA continuation and 3,809 to 5-ASA discontinuation. Compared to discontinuation, 5-ASA continuation was associated with decreased odds of achieving clinical remission (aOR 0.72; 95% CI 0.52-0.99; p=0.046; I2=0%). There were no significant differences between groups for corticosteroid-free clinical remission (aOR 0.71; 95% CI 0.41-1.23; p=0.22; I2=0%), clinical response (aOR 0.94; 95% CI 0.69-1.28; p=0.70; I2=0%), endoscopic healing (OR 0.96; 95% CI 0.64-1.44; p=0.85; I2=61%), and biochemical remission (aOR 1.13; 95% CI 0.76-1.68; p=0.54; I2=31%). In time-to-event analyses, no significant differences were found between groups for UC-related hospitalization (aHR 1.08; 95% CI 0.89-1.31; p=0.42; I2=51%), UC-related surgery (aHR 0.90; 95% CI 0.71-1.15; p=0.41; I2=36%), new corticosteroid prescription (aHR 0.92; 95% CI 0.79-1.06; p=0.24; I2=77%), composite adverse events (aHR 0.96; 95% CI 0.87-1.06; p=0.43; I2=0%), and loss of response (aHR 0.90; 95% CI 0.76-1.07; p=0.25; I2=77%). ROBINS-I results found one study with a serious risk of bias and eleven with a moderate risk of bias.
Discussion: In this meta-analysis of adjusted effect estimates, 5-ASA continuation was associated with a decreased odds of achieving clinical remission compared with 5-ASA discontinuation, with no significant differences observed for other efficacy and safety endpoints. These findings suggest that 5-ASA may be safely discontinued after escalation to advanced therapies in this population.

Figure: Efficacy Endpoints

Figure: Safety Endpoints - Time-to-Event Analysis
Disclosures:
Hyun Hee Sul indicated no relevant financial relationships.
Douglas Gewehr indicated no relevant financial relationships.
Hara Kang indicated no relevant financial relationships.
Odery Ramos jr indicated no relevant financial relationships.
Hyun Hee Sul, MD1, Douglas Mesadri. Gewehr, MD2, Hara Kang, MD3, Odery Ramos jr, PhD4. P5395 - Efficacy of 5-ASA Continuation versus Discontinuation in Patients With Moderate to Severe Ulcerative Colitis Escalated to Advanced Therapy: A Systematic Review and Meta-Analysis of Adjusted Effect Estimates, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.

