Tuesday Poster Session
Category: IBD
P5391 - Prospective Evaluation of Adherence to Early Clinical and Biochemical Assessments After Therapy Adjustment for Inflammatory Bowel Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Alec Mason, MD (he/him/his)
University of Texas Southwestern Medical Center
Dallas, TX
Presenting Author(s)
Alec Mason, MD, Noelle Cordova, PharmD, Erandi Santacruz, , Chelsea Missi, MD, Mary-Joe Touma, MD, Yassin El-Najjar, MD, Laura Pontes, PA-C, Shreeju Shrestha, APN, Moheb Boktor, MD, David Fudman, MD
University of Texas Southwestern Medical Center, Dallas, TX
Introduction: The treat-to-target (TTT) approach emphasizes time-based targets, however real-world adherence to early clinical and objective assessments after treatment adjustment is not well-described.
Methods: This single center prospective cohort study included patients starting a new advanced therapy (AT), escalating current AT, or reinducing current AT for ulcerative colitis (UC) or Crohn’s disease (CD) from 8/1/2024 to 12/15/2024. The dates of decision for therapy adjustment and first doses were recorded prospectively; additional data were collected retrospectively. Data were collected in conjunction with IBD Qorus TTT improvement efforts. Primary outcomes were completion of clinical (office or video visit) or biochemical (C-reactive protein [CRP] or fecal calprotectin [FCP]) assessments within 16 weeks of treatment adjustment date. Assessments < 2 weeks from this date were excluded.
Results: 148 patients underwent treatment adjustment (49% UC, 51% CD; Table 1A). Median time to first dose was 28 days (n=148, IQR 19-37), and was numerically shorter for single AT (26 days, n=86, IQR 15-35) than combination AT (29 days, n=20, IQR 20-44) and re-inductions/escalations (31 days, n=42, IQR 21-36). 81% (n=120) had on-time clinical assessments. On-time biochemical follow-up was less common, with 62% (n=91) having any on-time biomarker (for CRP, n=74 [50%]; for FCP n=58 [39.1%]). Both clinical and biochemical (either CRP or FCP) assessment was performed on time in 59% (n=87), falling to 27% (n=40) if considering completion of both biomarkers. In multivariate logistic regression (Table 1B), new starts were more likely to have clinical follow-up (aOR 2.90, 95% CI 1.08-7.78) and Hispanics less likely (aOR 0.25, 95% CI 0.07-0.94). Older age (aOR per year 0.97, 95% CI 0.94-0.99) and longer time from decision to start (aOR per day 0.97, 95% CI 0.95-0.99) were associated with lower odds of biochemical follow-up. Non-infusion induction and corticosteroid use were associated with higher odds of biochemical follow-up in univariable but not multivariable analysis.
Discussion: In this prospective single-center study, 1/5 of patients did not undergo clinical assessment and over 1/3 lacked biochemical assessment within 16 weeks of actual start date for a new AT or new AT dosing. If low rates of early TTT assessments are confirmed with multicenter data, adherence to these assessments could prove to be a more impactful quality improvement focus than later targets such as endoscopy.
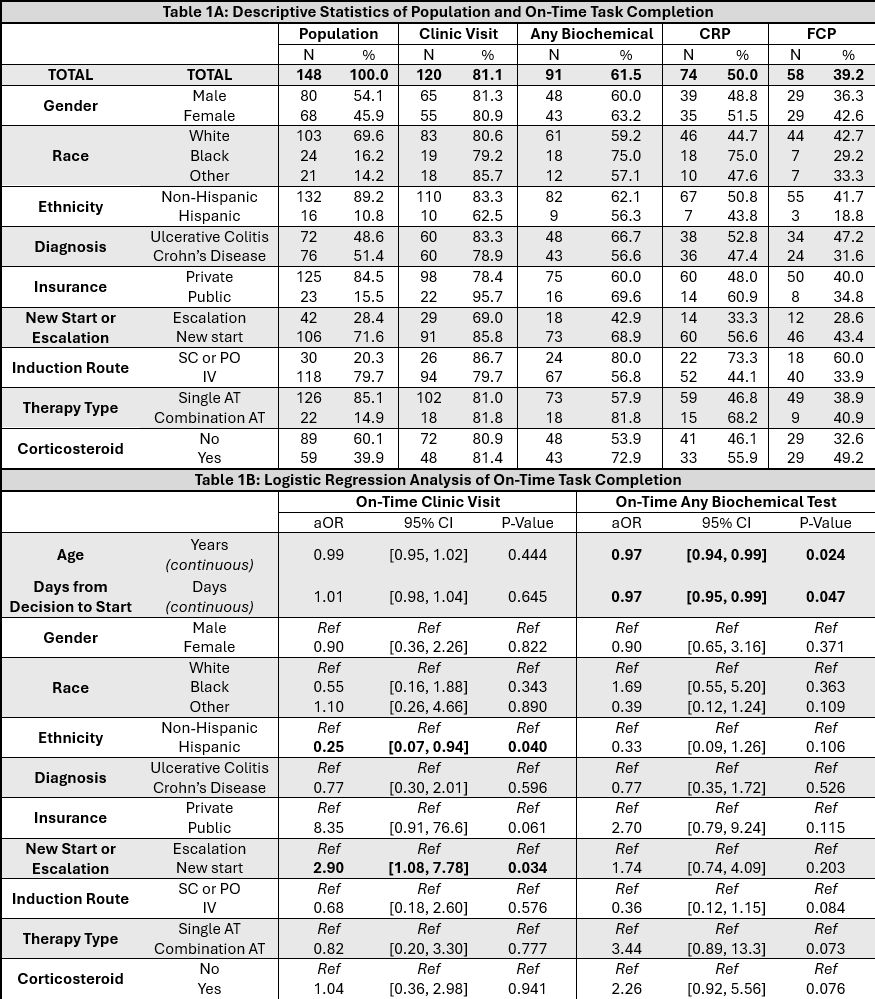
Figure: Table 1: Part (A) shows population descriptive statistics. The first column shows the distribution of variables in the study population, with percents relative to the total number of patients included in the study. The latter four columns show the number and percent of patients within each covariate group who completed the task within 2-16 weeks of their start date. Part (B) shows the results of a multivariable logistic regression for factors associated with on-time completion of appointments and any biochemical test, respectively. Corticosteroid prescriptions were considered active if unexpired with fills covering any date within the 30 days prior to decision date.
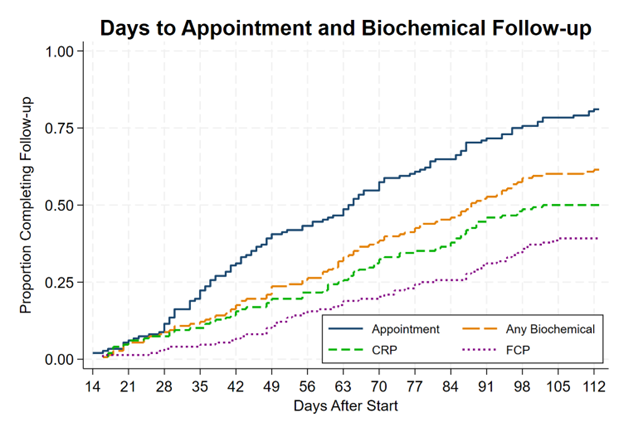
Figure: Figure 1: Kaplan-Meier curves showing days from start date to clinic appointment and biochemical follow-up. Follow-up task completions prior to 14 days after start date were excluded from this study.
Disclosures:
Alec Mason indicated no relevant financial relationships.
Noelle Cordova indicated no relevant financial relationships.
Erandi Santacruz indicated no relevant financial relationships.
Chelsea Missi indicated no relevant financial relationships.
Mary-Joe Touma indicated no relevant financial relationships.
Yassin El-Najjar indicated no relevant financial relationships.
Laura Pontes: Janssen – Advisory Committee/Board Member, Consultant.
Shreeju Shrestha indicated no relevant financial relationships.
Moheb Boktor indicated no relevant financial relationships.
David Fudman: Eli Lilly – Advisory Committee/Board Member. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member.
Alec Mason, MD, Noelle Cordova, PharmD, Erandi Santacruz, , Chelsea Missi, MD, Mary-Joe Touma, MD, Yassin El-Najjar, MD, Laura Pontes, PA-C, Shreeju Shrestha, APN, Moheb Boktor, MD, David Fudman, MD. P5391 - Prospective Evaluation of Adherence to Early Clinical and Biochemical Assessments After Therapy Adjustment for Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
University of Texas Southwestern Medical Center, Dallas, TX
Introduction: The treat-to-target (TTT) approach emphasizes time-based targets, however real-world adherence to early clinical and objective assessments after treatment adjustment is not well-described.
Methods: This single center prospective cohort study included patients starting a new advanced therapy (AT), escalating current AT, or reinducing current AT for ulcerative colitis (UC) or Crohn’s disease (CD) from 8/1/2024 to 12/15/2024. The dates of decision for therapy adjustment and first doses were recorded prospectively; additional data were collected retrospectively. Data were collected in conjunction with IBD Qorus TTT improvement efforts. Primary outcomes were completion of clinical (office or video visit) or biochemical (C-reactive protein [CRP] or fecal calprotectin [FCP]) assessments within 16 weeks of treatment adjustment date. Assessments < 2 weeks from this date were excluded.
Results: 148 patients underwent treatment adjustment (49% UC, 51% CD; Table 1A). Median time to first dose was 28 days (n=148, IQR 19-37), and was numerically shorter for single AT (26 days, n=86, IQR 15-35) than combination AT (29 days, n=20, IQR 20-44) and re-inductions/escalations (31 days, n=42, IQR 21-36). 81% (n=120) had on-time clinical assessments. On-time biochemical follow-up was less common, with 62% (n=91) having any on-time biomarker (for CRP, n=74 [50%]; for FCP n=58 [39.1%]). Both clinical and biochemical (either CRP or FCP) assessment was performed on time in 59% (n=87), falling to 27% (n=40) if considering completion of both biomarkers. In multivariate logistic regression (Table 1B), new starts were more likely to have clinical follow-up (aOR 2.90, 95% CI 1.08-7.78) and Hispanics less likely (aOR 0.25, 95% CI 0.07-0.94). Older age (aOR per year 0.97, 95% CI 0.94-0.99) and longer time from decision to start (aOR per day 0.97, 95% CI 0.95-0.99) were associated with lower odds of biochemical follow-up. Non-infusion induction and corticosteroid use were associated with higher odds of biochemical follow-up in univariable but not multivariable analysis.
Discussion: In this prospective single-center study, 1/5 of patients did not undergo clinical assessment and over 1/3 lacked biochemical assessment within 16 weeks of actual start date for a new AT or new AT dosing. If low rates of early TTT assessments are confirmed with multicenter data, adherence to these assessments could prove to be a more impactful quality improvement focus than later targets such as endoscopy.

Figure: Table 1: Part (A) shows population descriptive statistics. The first column shows the distribution of variables in the study population, with percents relative to the total number of patients included in the study. The latter four columns show the number and percent of patients within each covariate group who completed the task within 2-16 weeks of their start date. Part (B) shows the results of a multivariable logistic regression for factors associated with on-time completion of appointments and any biochemical test, respectively. Corticosteroid prescriptions were considered active if unexpired with fills covering any date within the 30 days prior to decision date.

Figure: Figure 1: Kaplan-Meier curves showing days from start date to clinic appointment and biochemical follow-up. Follow-up task completions prior to 14 days after start date were excluded from this study.
Disclosures:
Alec Mason indicated no relevant financial relationships.
Noelle Cordova indicated no relevant financial relationships.
Erandi Santacruz indicated no relevant financial relationships.
Chelsea Missi indicated no relevant financial relationships.
Mary-Joe Touma indicated no relevant financial relationships.
Yassin El-Najjar indicated no relevant financial relationships.
Laura Pontes: Janssen – Advisory Committee/Board Member, Consultant.
Shreeju Shrestha indicated no relevant financial relationships.
Moheb Boktor indicated no relevant financial relationships.
David Fudman: Eli Lilly – Advisory Committee/Board Member. Fresenius Kabi – Consultant. Janssen – Advisory Committee/Board Member.
Alec Mason, MD, Noelle Cordova, PharmD, Erandi Santacruz, , Chelsea Missi, MD, Mary-Joe Touma, MD, Yassin El-Najjar, MD, Laura Pontes, PA-C, Shreeju Shrestha, APN, Moheb Boktor, MD, David Fudman, MD. P5391 - Prospective Evaluation of Adherence to Early Clinical and Biochemical Assessments After Therapy Adjustment for Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
