Tuesday Poster Session
Category: IBD
P5376 - GLP-1 Receptor Agonists Decrease the Colorectal Cancer Rates in Inflammatory Bowel Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall
- KA
Khaled Alsabbagh Alchirazi, MD
Aurora Health Care
Brookfield, WI
Presenting Author(s)
Khaled Alsabbagh Alchirazi, MD1, Muaz Alsabbagh, MD2, Thabet Qapaja, MD3, Kinan Obeidat, MD4, Nishkarsh Kumar, 5, Bisher Sawaf, MD6, Miguel Regueiro, MD7
1Aurora Health Care, Brookfield, WI; 2Detroit Medical Center/Wayne State University, Cleveland, OH; 3Washington University School of Medicine in St. Louis, St. Louis, MO; 4University of Texas Medical Branch, Santa Fe, TX; 5St. Luke's Hospital, Milwaukee, WI; 6University of Toledo Medical Center, Toledo, OH; 7Cleveland Clinic, Cleveland, OH
Introduction: Patients with inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, are at increased risk of developing colorectal cancer (CRC) due to chronic intestinal inflammation. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), primarily used for metabolic conditions, have demonstrated anti-inflammatory and modulating immune functions. This study aims to evaluate the association between GLP-1RA use and the risk of CRC in patients with IBD
Methods: We performed a retrospective cohort study using the TriNetX database. Adults with a diagnosis of IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), were included between 2005 and 2022. Patients with a prior history of colorectal cancer were excluded. Two groups were compared: patients who received GLP-1 receptor agonists (GLP-1RA) and those who did not. Propensity score matching (1:1) was used to balance age, sex, race, and common medical comorbidities, personal and family hx of cancer history IBD medications and disease activity. The main outcome was new diagnosis of colorectal cancer. We used Cox regression to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). A p-value less than 0.05 was considered statistically significant
Results: The study included a total of 19,363 per group patients with IBD, matched 1:1 into GLP-1RA users and non-users. Patients exposed to GLP-1 receptor agonists demonstrated a significantly lower risk of developing CRC compared to non-users (38 vs. 87 cases; HR 0.39; 95% CI, 0.27–0.57; P < 0.001). Subgroup analysis revealed consistent findings across IBD subtypes. In patients with CD (n = 11,755 per group), GLP-1RA users had fewer CRC diagnoses than controls (23 vs. 48 cases; HR 0.44; 95% CI, 0.27–0.72; P = 0.001). Similarly, in patients with UC (n = 19,110 per group), those treated with GLP-1RAs had lower CRC incidence than non-users (51 vs. 88 cases; HR 0.51; 95% CI, 0.36–0.72; P < 0.001)
Discussion: GLP-1RA use was significantly associated with reduced CRC risk in patients with IBD, including both CD and UC. These findings support a potential protective effect, possibly linked to the anti-inflammatory and antineoplastic properties of GLP-1RAs. Given the elevated CRC risk in IBD, GLP-1RAs may offer dual benefits in this population—addressing metabolic disease while lowering cancer risk. Prospective studies are warranted to confirm these results and elucidate underlying mechanisms
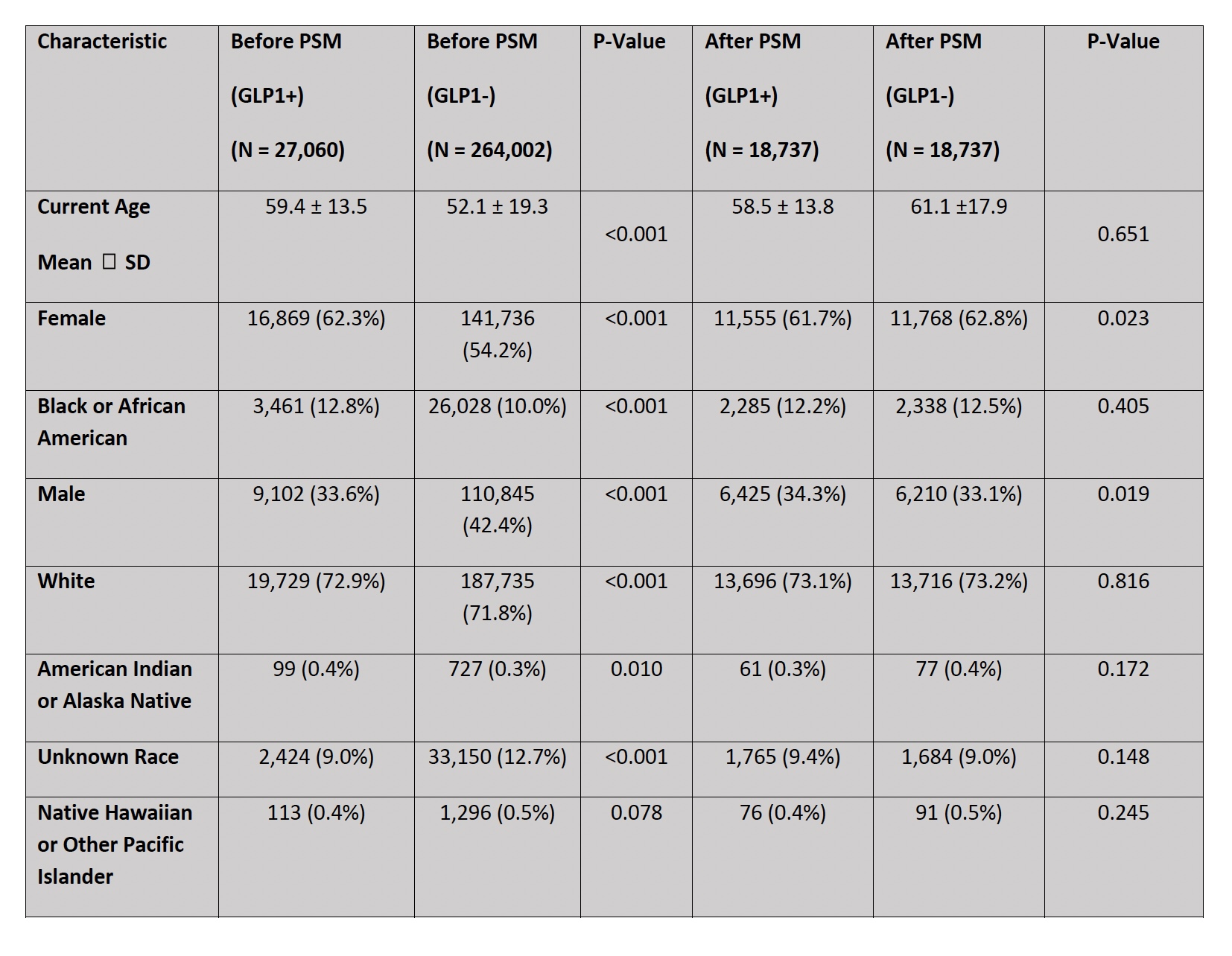
Figure: Table1: General Characteristics Before and After Propensity Score Matching
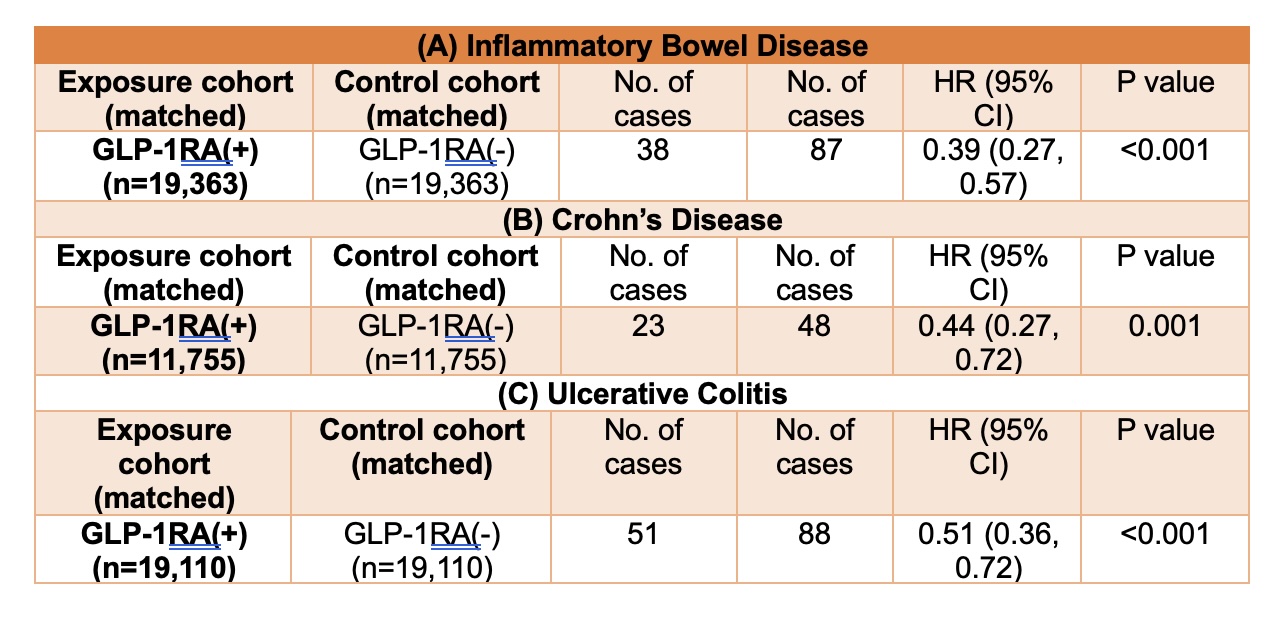
Figure: Table 2: Risks and Hazard Ratios (HRs) of First-Time Diagnosis of Colorectal Cancer (CRC) in Drug-Naive Patients With (A) Crohn's disease and (B) Ulcerative Colitis :
Disclosures:
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Muaz Alsabbagh indicated no relevant financial relationships.
Thabet Qapaja indicated no relevant financial relationships.
Kinan Obeidat indicated no relevant financial relationships.
Nishkarsh Kumar indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. BMS – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim Pharmaceuticals Inc. – Advisory Committee/Board Member, Consultant. Celegene – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant.
Khaled Alsabbagh Alchirazi, MD1, Muaz Alsabbagh, MD2, Thabet Qapaja, MD3, Kinan Obeidat, MD4, Nishkarsh Kumar, 5, Bisher Sawaf, MD6, Miguel Regueiro, MD7. P5376 - GLP-1 Receptor Agonists Decrease the Colorectal Cancer Rates in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Aurora Health Care, Brookfield, WI; 2Detroit Medical Center/Wayne State University, Cleveland, OH; 3Washington University School of Medicine in St. Louis, St. Louis, MO; 4University of Texas Medical Branch, Santa Fe, TX; 5St. Luke's Hospital, Milwaukee, WI; 6University of Toledo Medical Center, Toledo, OH; 7Cleveland Clinic, Cleveland, OH
Introduction: Patients with inflammatory bowel disease (IBD), including Crohn’s disease and ulcerative colitis, are at increased risk of developing colorectal cancer (CRC) due to chronic intestinal inflammation. Glucagon-like peptide-1 receptor agonists (GLP-1RAs), primarily used for metabolic conditions, have demonstrated anti-inflammatory and modulating immune functions. This study aims to evaluate the association between GLP-1RA use and the risk of CRC in patients with IBD
Methods: We performed a retrospective cohort study using the TriNetX database. Adults with a diagnosis of IBD, including Crohn’s disease (CD) and ulcerative colitis (UC), were included between 2005 and 2022. Patients with a prior history of colorectal cancer were excluded. Two groups were compared: patients who received GLP-1 receptor agonists (GLP-1RA) and those who did not. Propensity score matching (1:1) was used to balance age, sex, race, and common medical comorbidities, personal and family hx of cancer history IBD medications and disease activity. The main outcome was new diagnosis of colorectal cancer. We used Cox regression to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). A p-value less than 0.05 was considered statistically significant
Results: The study included a total of 19,363 per group patients with IBD, matched 1:1 into GLP-1RA users and non-users. Patients exposed to GLP-1 receptor agonists demonstrated a significantly lower risk of developing CRC compared to non-users (38 vs. 87 cases; HR 0.39; 95% CI, 0.27–0.57; P < 0.001). Subgroup analysis revealed consistent findings across IBD subtypes. In patients with CD (n = 11,755 per group), GLP-1RA users had fewer CRC diagnoses than controls (23 vs. 48 cases; HR 0.44; 95% CI, 0.27–0.72; P = 0.001). Similarly, in patients with UC (n = 19,110 per group), those treated with GLP-1RAs had lower CRC incidence than non-users (51 vs. 88 cases; HR 0.51; 95% CI, 0.36–0.72; P < 0.001)
Discussion: GLP-1RA use was significantly associated with reduced CRC risk in patients with IBD, including both CD and UC. These findings support a potential protective effect, possibly linked to the anti-inflammatory and antineoplastic properties of GLP-1RAs. Given the elevated CRC risk in IBD, GLP-1RAs may offer dual benefits in this population—addressing metabolic disease while lowering cancer risk. Prospective studies are warranted to confirm these results and elucidate underlying mechanisms

Figure: Table1: General Characteristics Before and After Propensity Score Matching

Figure: Table 2: Risks and Hazard Ratios (HRs) of First-Time Diagnosis of Colorectal Cancer (CRC) in Drug-Naive Patients With (A) Crohn's disease and (B) Ulcerative Colitis :
Disclosures:
Khaled Alsabbagh Alchirazi indicated no relevant financial relationships.
Muaz Alsabbagh indicated no relevant financial relationships.
Thabet Qapaja indicated no relevant financial relationships.
Kinan Obeidat indicated no relevant financial relationships.
Nishkarsh Kumar indicated no relevant financial relationships.
Bisher Sawaf indicated no relevant financial relationships.
Miguel Regueiro: AbbVie – Advisory Committee/Board Member, Consultant. Amgen – Advisory Committee/Board Member, Consultant. BMS – Advisory Committee/Board Member, Consultant. Boehringer Ingelheim Pharmaceuticals Inc. – Advisory Committee/Board Member, Consultant. Celegene – Advisory Committee/Board Member, Consultant. Eli Lilly and Company – Advisory Committee/Board Member, Consultant. Genentech – Advisory Committee/Board Member, Consultant. Gilead – Advisory Committee/Board Member, Consultant. Janssen – Advisory Committee/Board Member, Consultant. Organon – Advisory Committee/Board Member, Consultant. Pfizer – Advisory Committee/Board Member, Consultant. Prometheus – Advisory Committee/Board Member, Consultant. Salix – Advisory Committee/Board Member, Consultant. Takeda – Advisory Committee/Board Member, Consultant. UCB – Advisory Committee/Board Member, Consultant.
Khaled Alsabbagh Alchirazi, MD1, Muaz Alsabbagh, MD2, Thabet Qapaja, MD3, Kinan Obeidat, MD4, Nishkarsh Kumar, 5, Bisher Sawaf, MD6, Miguel Regueiro, MD7. P5376 - GLP-1 Receptor Agonists Decrease the Colorectal Cancer Rates in Inflammatory Bowel Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
