Tuesday Poster Session
Category: IBD
P5371 - Further Validation of the Vedolizumab Clinical Decision Support Tool to Predict the Likelihood of Clinical Remission in Vedolizumab-Treated Patients With Crohn’s Disease
Tuesday, October 28, 2025
10:30 AM - 4:00 PM PDT
Location: Exhibit Hall

Christian Agboton, MD
Takeda Pharmaceuticals
Cambridge, MA
Presenting Author(s)
Marlies Meyer, MD1, Dirk Lindner, MSc1, Christian Agboton, MD2, Paul Talsma, 3
1Takeda Pharmaceuticals AG, Zurich, Zurich, Switzerland; 2Takeda Pharmaceuticals, Cambridge, MA; 3Vivos Technology Limited (Phastar), London, England, United Kingdom
Introduction: Despite multiple treatment options, therapeutic management of Crohn’s Disease (CD) can be challenging. Vedolizumab (VDZ) is a gut-specific anti-lymphocyte trafficking agent approved for the treatment of CD. Clinical decision support tools can help inform clinicians’ decisions regarding therapeutic management. The Vedolizumab Clinical Decision Support Tool (VDZ-CDST) categorizes patients (pts) as having a low, intermediate or high probability of response to VDZ treatment after 26 weeks based on 5 baseline parameters: prior anti-TNF exposure, prior bowel surgery, prior fistulizing disease, albumin and C-reactive protein.
Methods: This post-hoc analysis aimed to further validate the VDZ-CDST using pooled data from the Phase 3 GEMINI 2 (NCT00783692), VISIBLE 2 (NCT02611817) and VERSIFY (NCT02425111) clinical trials. The primary endpoint was the concordance index (c-index) of the VDZ-CDST score in discriminating pts achieving and not achieving CREM (Crohn’s Disease Activity Index score ≤150) at W26. Secondary endpoints included CREM rates at W26 in the 3 response probability groups, and sensitivity, specificity, positive and negative predictive values for both VDZ-CDST cut-offs (low vs intermediate/high and low/intermediate vs high).
Results: This analysis included pooled data from 1186 pts with CD who received VDZ intravenous (813 from GEMINI 2, 275 from VISIBLE 2 and 98 from VERSIFY; 50.8% female, median age 34 years, median disease duration 7.5 years, 63.5% with prior TNF exposure), of whom the VDZ-CDST categorised 240 as having a low, 547 having an intermediate and 399 having a high probability of response to VDZ treatment. The c-index (95% CI) for CREM at W26 was 0.714 (0.682-0.744), indicating reasonable discriminant ability. The rates of CREM at W26 in the low, intermediate and high probability groups were 10.4%, 23.9% and 49.4%, respectively, showing a positive linear trend (p< 0.0001) between the VDZ-CDST response probability groups. For low/intermediate vs high, sensitivity was 55.8% (50.6-60.9) and specificity was 75.8% (72.7-78.5; Table).
Discussion: This analysis further supports the ability of VDZ-CDST to discriminate between pts on VDZ treatment with and without CREM at W26, with a linear decrease in CREM W26 remission rates from the high probability group to the intermediate and low probability groups. Findings further validate the utility of the VDZ-CDST in routine clinical practice as part of a stratified approach to treatment.
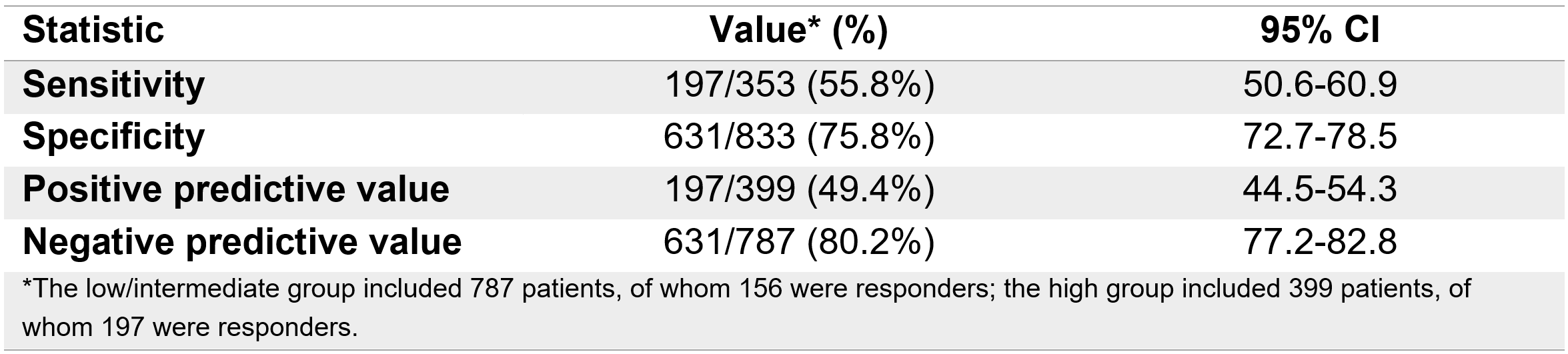
Figure: Table. Sensitivity, specificity, positive predictive value and negative predictive value of the VDZ-CDST for low/intermediate vs high
Disclosures:
Marlies Meyer: Takeda – Employee, Stock Options.
Dirk Lindner: Takeda – Employee, Stock Options.
Christian Agboton: Takeda – Employee, Stock Options.
Paul Talsma: Takeda Development Centre Europe Ltd – Consultant.
Marlies Meyer, MD1, Dirk Lindner, MSc1, Christian Agboton, MD2, Paul Talsma, 3. P5371 - Further Validation of the Vedolizumab Clinical Decision Support Tool to Predict the Likelihood of Clinical Remission in Vedolizumab-Treated Patients With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
1Takeda Pharmaceuticals AG, Zurich, Zurich, Switzerland; 2Takeda Pharmaceuticals, Cambridge, MA; 3Vivos Technology Limited (Phastar), London, England, United Kingdom
Introduction: Despite multiple treatment options, therapeutic management of Crohn’s Disease (CD) can be challenging. Vedolizumab (VDZ) is a gut-specific anti-lymphocyte trafficking agent approved for the treatment of CD. Clinical decision support tools can help inform clinicians’ decisions regarding therapeutic management. The Vedolizumab Clinical Decision Support Tool (VDZ-CDST) categorizes patients (pts) as having a low, intermediate or high probability of response to VDZ treatment after 26 weeks based on 5 baseline parameters: prior anti-TNF exposure, prior bowel surgery, prior fistulizing disease, albumin and C-reactive protein.
Methods: This post-hoc analysis aimed to further validate the VDZ-CDST using pooled data from the Phase 3 GEMINI 2 (NCT00783692), VISIBLE 2 (NCT02611817) and VERSIFY (NCT02425111) clinical trials. The primary endpoint was the concordance index (c-index) of the VDZ-CDST score in discriminating pts achieving and not achieving CREM (Crohn’s Disease Activity Index score ≤150) at W26. Secondary endpoints included CREM rates at W26 in the 3 response probability groups, and sensitivity, specificity, positive and negative predictive values for both VDZ-CDST cut-offs (low vs intermediate/high and low/intermediate vs high).
Results: This analysis included pooled data from 1186 pts with CD who received VDZ intravenous (813 from GEMINI 2, 275 from VISIBLE 2 and 98 from VERSIFY; 50.8% female, median age 34 years, median disease duration 7.5 years, 63.5% with prior TNF exposure), of whom the VDZ-CDST categorised 240 as having a low, 547 having an intermediate and 399 having a high probability of response to VDZ treatment. The c-index (95% CI) for CREM at W26 was 0.714 (0.682-0.744), indicating reasonable discriminant ability. The rates of CREM at W26 in the low, intermediate and high probability groups were 10.4%, 23.9% and 49.4%, respectively, showing a positive linear trend (p< 0.0001) between the VDZ-CDST response probability groups. For low/intermediate vs high, sensitivity was 55.8% (50.6-60.9) and specificity was 75.8% (72.7-78.5; Table).
Discussion: This analysis further supports the ability of VDZ-CDST to discriminate between pts on VDZ treatment with and without CREM at W26, with a linear decrease in CREM W26 remission rates from the high probability group to the intermediate and low probability groups. Findings further validate the utility of the VDZ-CDST in routine clinical practice as part of a stratified approach to treatment.

Figure: Table. Sensitivity, specificity, positive predictive value and negative predictive value of the VDZ-CDST for low/intermediate vs high
Disclosures:
Marlies Meyer: Takeda – Employee, Stock Options.
Dirk Lindner: Takeda – Employee, Stock Options.
Christian Agboton: Takeda – Employee, Stock Options.
Paul Talsma: Takeda Development Centre Europe Ltd – Consultant.
Marlies Meyer, MD1, Dirk Lindner, MSc1, Christian Agboton, MD2, Paul Talsma, 3. P5371 - Further Validation of the Vedolizumab Clinical Decision Support Tool to Predict the Likelihood of Clinical Remission in Vedolizumab-Treated Patients With Crohn’s Disease, ACG 2025 Annual Scientific Meeting Abstracts. Phoenix, AZ: American College of Gastroenterology.
